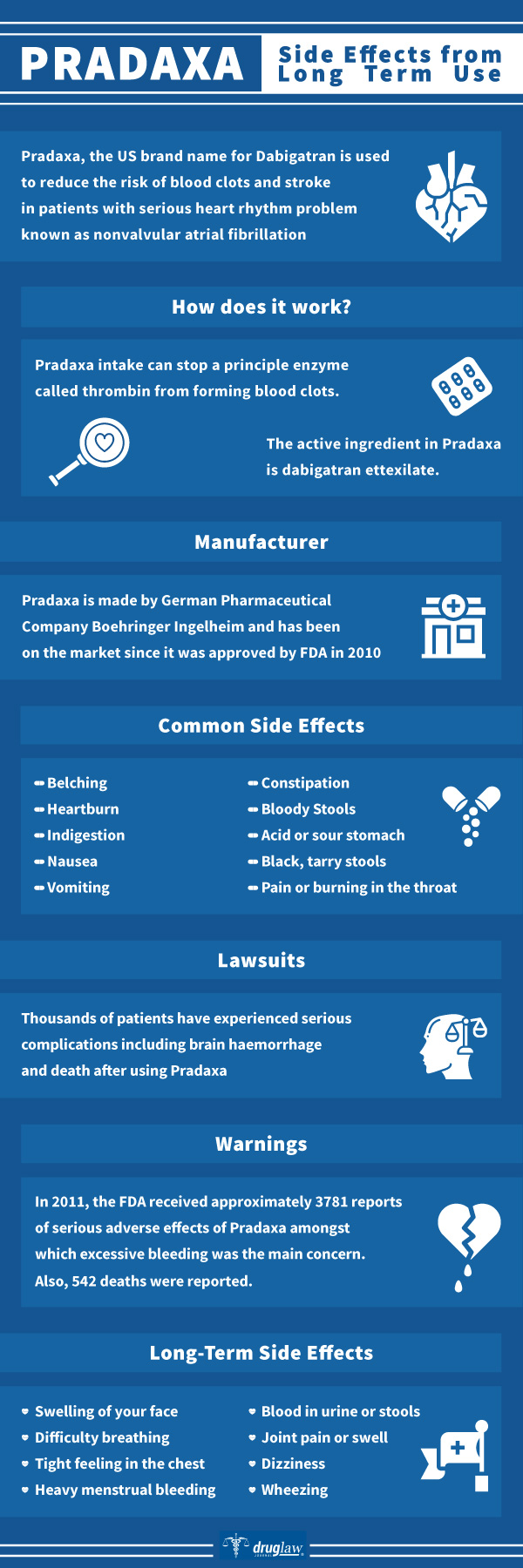
Pradaxa, the brand name for Dabigatran, is used to reduce the risk of blood clots and stroke in patients with serious heart rhythm problems, known as nonvalvular atrial fibrillation. It is also used to prevent harmful blood clots in the lungs or legs. Dabigatran is used to treat and prevent blood clots; which can be present in the veins of your leg (deep vein thrombosis), lungs (pulmonary embolism), etc. Pradaxa reduces the risk of these clots in patients who have already received other medications.
Pradaxa is an anticoagulant that works by blocking a certain substance called thrombin in your blood. This supports the steady flow of blood in the body. Pradaxa should not be used solely to prevent the formation of blood clots after surgeries, such as artificial heart valve replacement. If you have had this surgery, do not take this or any other medicine without consulting your doctor.
Prescription Name and Overview
Pradaxa (Dabigatran) is mainly used to reduce blood clot formation in the veins of your legs or lungs. It also reduces the risk f stroke in people with a certain type of heart rhythm disorder. As an oral dosage, Pradaxa capsules are prescribed in 75, 110 and 150mg, depending on the patient’s condition. The capsule shell is composed of carrageenan, potassium chloride, hypromellose, black edible ink and titanium dioxide.
Generic Name and Overview
Pradaxa is the brand name for the generic compound called Dabigatran. This medicine is generally prescribed for treating pulmonary embolism and deep vein thrombosis. Dabigatran is also prescribed to patients, who are at the initial state of developing blood clots. Patients who recently had hip surgery or individuals with atrial fibrillation or a heart condition that may lead to clot formation are, at times, prescribed Pradaxa. The main reason for prescribing Pradaxa is to prevent death from a heart attack or a stroke.
OTC Name and Overview
Pradaxa (Dabigatran) is used to treat deep vein thrombosis (DVT) and pulmonary embolism (PE) in patients who have received treatment with an injectable anticoagulant (‘blood thinner’). This drug helps to reduce the risk of PE And DVT from occurring again. Pradaxa is also used to help prevent serious blood clots and stroke in patients with atrial fibrillation. It is a class of anticoagulant medication called thrombin inhibitors. Dabigatran is usually taken one to four hours after the surgery and then once per day for another 28 to 35 days. Dosage depends on the individual and should never be taken without consulting your doctor.
Manufacturer
Pradaxa (Dabigatran) is a blood thinner used to cure blood clots and to reduce the risk of strokes or heart attacks in individuals prone to the formation of clots. This drug is manufactured by German Pharmaceutical Company, Boehringer Ingelheim and has been on the market since it was approved by the United States Food and Drug Administration (FDA) in 2010. Along with other blood thinners, Pradaxa was created as a safer substitute to Warfarin, which has been used as a blood thinner for decades. Primarily, Pradaxa helps in the prevention of venous thromboembolic (VTE) in adults after hip or knee replacement surgery. It is also used in the treatment of deep vein thrombosis and pulmonary embolism and secondary prevention of recurring events of these disorders in adults.
Labeled Indications
- To reduce the risk of stroke and systemic embolism in non-valvular atrial fibrillation (AF)
- Primary prevention of venous thromboembolic after orthopedic surgery, such as a knee replacement or hip replacement
- Treatment for prevention for recurrence of these disorders
- Prophylaxis of deep vein thrombosis (DVT) and pulmonary embolism (PE)
Active Ingredients
The active ingredient in Pradaxa is Dabigatran Etexilate Mesilate. After oral usage, Dabigatran Etexilate is rapidly converted in the body to its active form Dabigatran. This ingredient is, at times, referred to as a ‘blood thinner’ that works by inhibiting a specific protein in the blood called thrombin. The dosage of Pradaxa is normally prescribed in 75, 110 or 150 mg. The active ingredients in every dosage are as follows:
- Pradaxa 75 mg – 75 mg Dabigatran Etexilate given as 86.48 mg Dabigatran Etexilate Mesilate per capsule
- Pradaxa 110 mg – 110 mg Dabigatran Etexilate given as 126.83 mg Dabigatran Etexilate Mesilate per capsule
- Pradaxa 150 mg – 150 mg Dabigatran Etexilate given as 172.95 mg Dabigatran Etexilate Mesilate per capsule
What Is It Used For?
Pradaxa is a prescription drug that helps prevents the formation of blood clots in the veins after surgery. Patients with abnormal heartbeats are often asked to take Pradaxa to prevent the formation of blood clots and strokes. The following are some of the typical uses of Pradaxa:
- Prevent the formation of blood clots in the veins after hip or knee replacement surgery in adults
- Reduce the risk of stroke (brain) and/or other body vessel obstruction by blood clot formation in adults
- Prescribed to patients with abnormal heart rhythm, called non-valvular atrial fibrillation
- Treat blood clots in your lungs and veins to prevent blood clots from recurring in the veins of your lungs or legs
How Does it Work?
Pradaxa intake can stop a principle enzyme called thrombin from forming blood clots. Blood clots can lead to various deadly health conditions and risks; including deep vein thrombosis, pulmonary embolism and strokes.
What are the Approved Uses?
The approved uses of Pradaxa are to lower the risk of stroke caused by blood clots in people with atrial fibrillation and used after surgery to prevent deep vein thrombosis (DVT) a condition that can lead to blood clots in the lungs (pulmonary embolism).
Production Anecdotes / History
Pradaxa (Dabigatran) was discovered from a panel of chemicals with a similar structure to benzamidine-based thrombin which has been known since the 1980’s as a powerful inhibitor of various serine proteases, especially thrombin. The European Medicines Agency granted marketing authorization of Pradaxa on March 2008 for the prevention of thromboembolic diseases following knee or hip replacement surgery and for non-valvular atrial fibrillation. This medicine was also authorized by Britain’s NHD for the prevention of blood clot formation in hip and knee surgery patients. The United States Food and Drug Administration (FDA) approved Pradaxa on October 19, 2010 for the prevention of stroke in patients with non-valvular trial and stroke.
Precautions
Before taking this medication it is important to consider the adverse effects associated with Pradaxa. It is always recommended you confer with your doctor before use. Before using Pradaxa, it is advised to share your medical history with your physician, especially in regards to a heart condition, kidney disease, bleeding problem (such as bleeding in the intestine/stomach), blood disorder (such as haemophilia, anaemia), any recent injury, surgery etc.
Stop or limit alcohol intake on a regular basis as it may increase the chances of stomach bleeding. Older adults may be at greater risk of bleeding during the usage of this medicine. During pregnancy, medication, including Pradaxa, should never be taken without consulting your doctor.
Long-Term Use Considerations
Pradaxa (Dabigatran) helps to reduce the risk of formation of blood clots; yet, as a blood thinner it is necessary that when you are taking it for an extended period of time, you are monitored by your physician or medical team. Unnecessary consumption of Pradaxa without a doctor’s advice may lead to adverse effects.
Drug Interactions
Do not start, stop or change the dosage of any medicines without the approval of your doctor.
Can Interact With the Following
Severe Interaction
These medications are not typically taken together. Check with your healthcare expert for more information.
- Anticoagulant; Antiplatelet; Thrombolytic/Alipogene Tiparvovec
- Anticoagulants; Antiplatelets/ Mifepristone
Serious Interaction
These medicines may interact and cause very harmful effects. Consult your doctor for more information.
- Dabigatran/Sulfinpyrazone
- Dabigatran/NSAIDs
- Dabigatran/P-Glycoprotein (P-gp) Inducers
- Dabigatran/Antiplatelets; Aspirin (> 100 Mg)
- Dabigatran/NSAIDs
- Dabigatran/Dronedarone, Ketoconazole
- Dabigatran/Anticoagulants; Dextran; Thrombolytics
- Anticoagulants; Antiplatelets/Inotersen
- Anticoagulants; Thrombolytics/Betrixaban
- Anticoagulants; Thrombolytics/Rivaroxaban
- Anticoagulants; Thrombolytics/Apixaban
- Dabigatran/Heparins
- Dabigatran/P-Glycoprotein (P-gp) Inducers
- Dabigatran/NSAIDs
Moderate Interactions
Consult your healthcare provider before taking this medicine as it may lead to some risk when taken together.
- Dabigatran/Aspirin (< Or = 100 Mg)
- Selected P-Glycoprotein (P-gp) Substrates/Ibrutinib
- Dabigatran/Selected P-Glycoprotein (P-gp) Inhibitors
- Dabigatran/Lovastatin; Simvastatin
- Dabigatran/Aspirin (< Or = 100 Mg)
- Dabigatran/Abciximab
- Selected P-Glycoprotein (P-gp) Substrates/Ibrutinib
- Slt Anticoagulants;Antiplatelets; Thrombolytics/Ssri’s;SNRI’s
- Apixaban; Dabigatran; Rivaroxaban/Fluconazole
When To Stop Taking
Your doctor should advise you when to stop taking Pradaxa. Pradaxa is a blood thinner that has been prescribed by doctors as a remedy to help prevent the formation of blood clot. However, if you have serious side effects with Pradaxa, your doctor may suggest you use other medications or blood thinners. It is not advisable to stop taking Pradaxa without informing your doctor as it may increase the risk of stroke.
Long-Term Side Effects
Pradaxa has been associated with different long and short-term side effects. It is necessary to get emergency medical help if you have signs of an allergic reaction to Pradaxa. Some of the long-term side effects are swelling of your face, difficulty breathing, tight feeling in the chest, wheezing etc.
It is also advised to seek emergency medical attention if you experience symptoms of a spinal blood clot, which can include numbness, back pain, or bowel problems.
Some additional side effects of Pradaxa are:
- Unstoppable bleeding
- Dizziness, headache
- Unusual bleeding (nose, mouth, rectum or vagina), purple or red spots under your skin
- Blood in urine or stools
- Easy bruising
- Cough with bloody mucus
- Joint pain or swell
- Heavy menstrual bleeding
FDA Warnings
Pradaxa was approved by the Food and Drug Administration (FDA) in October 2010. The drug has been regarded as a revolutionary replacement of Warfarin. Pradaxa has often been compared to Coumadin (Warfarin); the advantage of Pradaxa being it does not need as intensive monitoring or frequent blood tests. Despite Pradaxa’s commercial success, shortly after its success, serious concerns began to emerge about the drug’s safety. In 2011, the FDA received approximately 3,781 reports of serious adverse effects of Pradaxa among which excessive bleeding was the main concern. At this time, 542 deaths have been reported.
Other Common Side Effects
If you experience any of the following side effects, it is important for you to speak with your doctor. According to various peer-reviewed journals, some of the common side effects of Pradaxa are as follows:
- Acid or sour stomach
- Black, tarry stools
- Belching
- Constipation
- Heartburn
- Indigestion
- Nausea
- Bloody stools
- Stomach discomfort
- Pain or burning in the throat
- Vomiting
Lawsuits
Thousands of patients have experienced serious complications from Pradaxa, including brain hemorrhage and death. Between 2010 and 2013, patients or their loved ones who suffered from excessive bleeding while taking Pradaxa filed thousands of liability claims and lawsuits against the manufacturer of the drug, Boehringer Ingelheim. By 2014, the company had reached a settlement, but denied the wrongdoings that stated Pradaxa could lead to intestinal bleeding. Although these lawsuits were filed and eventually settled, Pradaxa’s manufacturers still claim there is no need to monitor patients taking Pradaxa.