Prescription Name and Overview
Truvada is the market name for a therapeutic medication to combat the Human Immunodeficiency Virus (HIV). It is actually a combination of two drugs: tenofovir disoproxil fumarate and emtricitabine that are used to treat and even prevent HIV infection. Truvada is also prescribed to treat hepatitis B infection. Truvada works by blocking important cellular pathways that HIV uses to further infection growth.
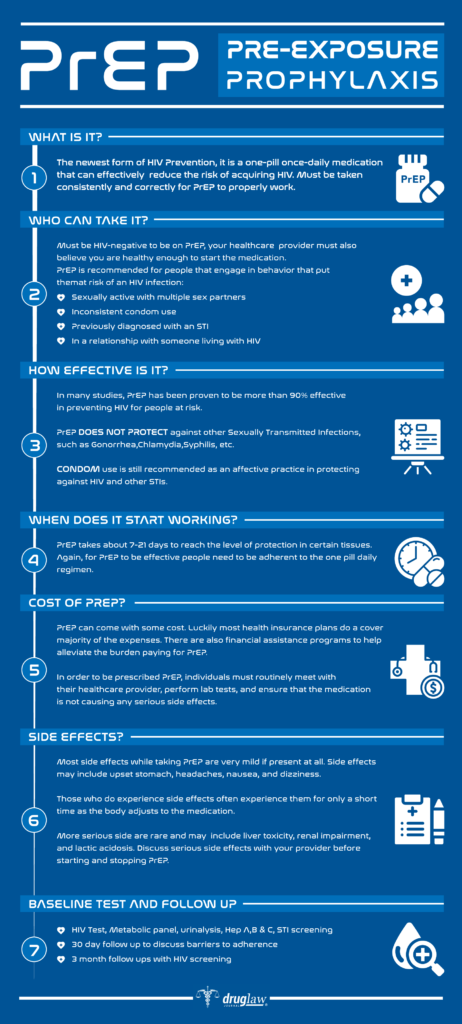
Truvada can be prescribed to people who are not HIV-positive, but who may not always use condoms or who have an HIV-positive sex partner, to reduce the potential for HIV infection. Taking Truvada to prevent HIV infection is known as “pre-exposure prophylaxis” or “PrEP”.
Gilead Sciences, Inc. developed Truvada with substantial research and development assistance from the National Institutes of Health. Truvada was approved by the U.S. Food and Drug Administration (FDA) as a treatment for HIV infection in August 2004 and in 2012 as a tool for reducing the risk of sexually transmitted HIV infection.
The FDA’s approved label for Truvada warns that frequent use of the medication may result in new-onset or worsening renal (kidney) impairment. Additionally, the FDA warns users and their physicians of the possibility of decreases in bone mineral density (BMD). Given Truvada’s widespread use and its adoption by younger men, advocates have warned about downplaying the risk Truvada poses to long-term health concerns. Recently, several Truvada patients have filed suit in court seeking compensation for their injuries which they believe stem from frequent use of Truvada.
Generic Name and Overview
Emtricitabine/Tenofovir Disoproxil Fumarate (TDF) tablets for oral use.
Manufacturer and Labeling Overview
Gilead Sciences, Inc.
Foster City, California
Labeled Indications
Truvada is a combination drug therapy using two nucleoside analog HIV-1 reverse transcriptase inhibitors. It is typically prescribed in combination with other antiretroviral therapies for the treatment of HIV-1 infections in adult and pediatric patients. Truvada can also be prescribed, in combination with safer-sex practices, to reduce the risk of sexually acquired HIV-1.
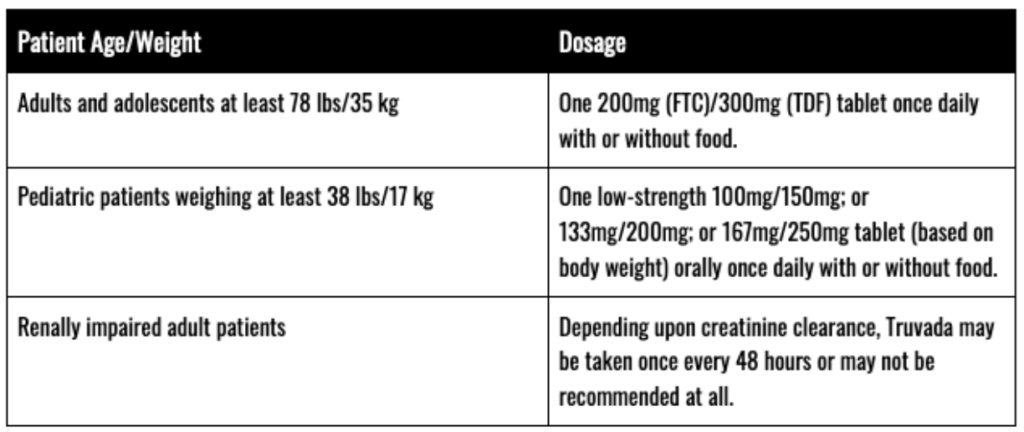
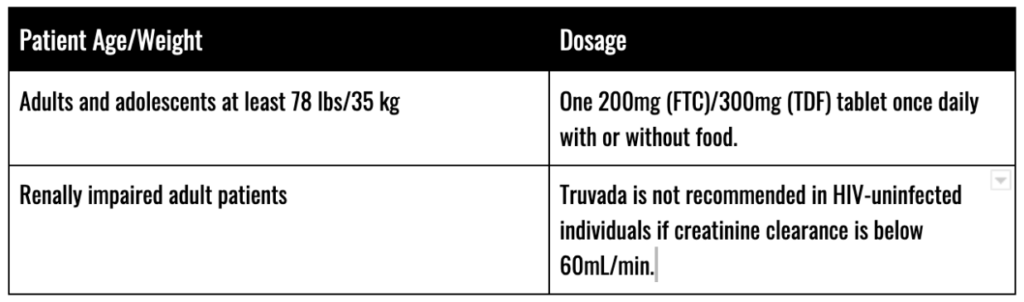
Typical Dosages
Prior to commencing a Truvada regimen, patients should be tested for the presence of chronic hepatitis B virus (HBV). Additionally, patients seeking to take Truvada for PrEP should be screened for HIV-1 infection before starting Truvada and then at least once every three (3) months while taking Truvada.
Before and during use of Truvada, patients should also be tested to assess:
- Serum Creatinine
- Estimated Creatinine Clearance
- Urine Glucose
- Urine Protein
- Serum Phosphorus (in patients with chronic kidney disease (CKD)
Treatment of HIV-1 Infection
HIV-1 PrEP
How Does It Work?
The two active drugs in Truvada work to block an enzyme known as reverse transcriptase which is used by the HIV-1 virus to copy itself and reproduce inside the body.
What Are the Approved Uses?
Truvada is approved for the treatment of HIV-1 infection in adults and pediatric patients in combination with other antiretroviral agents. It is also approved for use as PrEP in combination with safer sex practices in HIV-1 uninfected individuals. Truvada is also prescribed “off-label” as a treatment for hepatitis B virus (HBV) infection.
How Effective is PrEP?
According to the U.S. Centers for Disease Control (CDC), PrEP reduces the risk of contracting HIV from sexual activity up to 99% when taken on a daily basis. Additionally, PrEP is effective at reducing the risk of infection from intravenous drug use by at least 74%.
PrEP gives maximum protection for individuals engaging in receptive anal sex (bottoming) following seven (7) days of daily use. PrEP reaches maximum protection for receptive vaginal sex and intravenous drug use after twenty-one (21) days of daily use.
Production Anecdotes/History
Non-generic Truvada was first manufactured by Gilead Sciences. Gilead filed for approval from the FDA in 2001 for the antiretroviral drug TDF which forms one of the two crucial drugs in the Truvada regimen. At that time, it was sold as Viread and was part of a number of revolutionary drugs combating HIV infection. Although Viread was broadly considered safe and effective, its FDA-approved label made pointed references to issues with bone mineral density and the potential onset of new or worsening renal dysfunction (kidney impairment).
Following its approval for the treatment of HIV, the federal government through the National Institutes of Health (NIH) as well as the Bill and Melinda Gates Foundation-funded extensive research on Truvada’s effectiveness at preventing HIV infection. This public research into PrEP eventually revealed that a once-daily regimen of the combination of TDF and emtricitabine reduced new infections among mostly gay and bisexual men. Truvada was then approved for PrEP in 2012.
Non-generic Truvada is not an inexpensive drug. Prior to the expiration of its patent and the introduction of a generic equivalent, Gilead marketed Truvada for around $1,600 – $2,000 per month and it was responsible for about $3 billion in revenue for the drug firm during 2018 alone. Accordingly, Truvada’s expense/profitability on the heels of substantial public sector investment sparked considerable consternation and controversy. In 2019, activist groups and the CDC each filed lawsuits either seeking royalties or to enforce patents held by the U.S. Government on Truvada for PrEP. In response, Gilead sued the U.S. Government for breach of contract in 2020. These lawsuits are ongoing.
Tenofir Alafenamide (TAF) and Descovy
While Gilead was developing TDF as an antiretroviral drug, it was developing another similar drug on a parallel development track: tenofir alafenamide (TAF). TDF and TAF both showed exceptional promise at rolling back the progress of HIV-1 infection. However, TAF appeared to do so as effectively as TDF, just with a lower dosage and fewer side effects.
TDF went on to gain approval from the FDA first and Gilead rushed Viread and later Truvada onto the market making billions as the sole patent holder. At the same time, Gilead largely sidelined its research into TAF and did not submit it for FDA approval until the patent for TDF neared its expiration. The FDA approved Gilead’s new TAF PrEP combo Descovy in 2019, just over a year prior to the expiration of the Truvada patent in 2021. This gave Gilead enough time to engage in a marketing blitz to switch Truvada customers over to its newer, patent-protected medication, Descovy.
The submission timeline for TDF/Truvada and TAF/Descovy by Gilead has opened it up to accusations that the company knowingly “slow-rolled” research and FDA approval of a product (TAF/Descovy). This allowed Gilead to first fully take advantage of the revenue to be gained from the patent for TDF/Viread/Truvada, a product that requires a higher dosage and has demonstrably more serious side effects than TAF/FDescovy.
Generic Equivalent
In April 2021, multiple manufacturers, including Teva Pharmaceuticals, announced that they would be marketing generic versions of Truvada.
Precautions
Pregnancy
Data and post-marketing observations have not shown any increase in the risk of major birth defects when Truvada is used during pregnancy. There is a healthcare provider pregnancy exposure registry to monitor pregnancy outcomes in women exposed to Truvada during pregnancy.
Other Precautions
Truvada carries the following warnings and precautions:
- Severe Acute Exacerbation of Hepatitis B in Patients with HBV Infection
- New Onset or Worsening Renal Impairment
- Immune Reconstitution Syndrome
- Bone Loss Mineralization Defects
- Lactic Acidosis/Severe Hepatomegaly with Steatosis
Long-Term Use Considerations
Aside from the labeled long-term side effects and more common side effects, some research has indicated that long-term PrEP exposure may impact the levels of certain bacteria in the human digestive microbiome. A study recently published in Scientific Reports states that long-term PrEP exposure reduced the levels of microbiome bacteria Streptococcus while increasing levels of Erysipelotrichaceae.
Drug Interactions
Truvada may interact with other drugs that are may cause harm to the kidneys, such as:
- Adefovir
- Orlistat
- Amikacin/Gentamicin
Truvada may decrease the effectiveness of other medications such as:
- Atazanavir
Long Term Side Effects
Truvada may increase the risk of bone loss and patients should discuss this possibility with their physician. Patients taking Truvada should immediately contact their physician if they experience mental health change (depression/anxiety) or renal problems (changes in the amount of urine or pink/bloody urine).
Other Common Side Effects
Taking Truvada may involve the experience of side effects, including – but not limited to:
- Headache
- Nausea
- Vomiting
- Rash
- Loss of Appetite
- Creatinine and Transaminase Increase
- Lactic Acidosis
- Temporary Changes in Skin Color
FDA Warnings
Since its approval in 2001, the label for TDF has included warnings from the FDA about the risks associated with loss of bone mineral density and kidney damage. Gilead has been accused of downplaying these risks in its marketing for Truvada and the FDA itself has sent warning letters to Gilead about its aggressive marketing for Viread and Truvada.
Lawsuits
Individuals who were prescribed TDF/Viread/Truvada and who have suffered bone density loss, osteoporosis, brittle bones, and/or kidney injuries have recently filed lawsuits. These legal actions allege that Gilead knew that TDF was defective and that there was a safer alternative available (TAF). Furthermore, that Gilead failed to adequately warn doctors and their patients about the serious side effects presented by TDF/Viread/Truvada as well as downplaying the side effects in its own marketing of the drug.
Sources Cited (25)
1. “Pre-exposure Prophylaxis (PrEP) to Reduce HIV Risk” https://www.niaid.nih.gov/diseases-conditions/pre-exposure-prophylaxis-prep
2. “Long-acting injectable form of HIV prevention outperforms daily pill in NIH study” https://www.nih.gov/news-events/news-releases/long-acting-injectable-form-hiv-prevention-outperforms-daily-pill-nih-study
3. “Drug Database: Emtricitabine / Tenofovir Disoproxil Fumarate” https://clinicalinfo.hiv.gov/en/drugs/emtricitabine-tenofovir-disoproxil-fumarate/patient
4. “HIV Prevention: Pre-Exposure Prophylaxis (PrEP)” https://hivinfo.nih.gov/understanding-hiv/fact-sheets/pre-exposure-prophylaxis-prep
5. “What is PrEP?” https://www.hiv.gov/hiv-basics/hiv-prevention/using-hiv-medication-to-reduce-risk/pre-exposure-prophylaxis
6. “HIV-1 PrEP drug can be part of a strategy to prevent infection in at-risk adolescents” https://www.fda.gov/files/science%20&%20research/published/HIV-PrEP-drug-can-be-part-of-strategy-to-prevent-infection-in-at-risk-adolescents.pdf
7. “Pre-Exposure Prophylaxis (PrEP)” https://www.cdc.gov/hiv/clinicians/prevention/prep.html
8. “Truvada Medication Information Sheet for Patients” https://www.cdc.gov/hiv/pdf/prep_gl_patient_factsheet_truvada_english.pdf
9. “About PrEP” https://www.cdc.gov/hiv/basics/prep/about-prep.html
10. “Drug-Company Patents vs. the Public Good: Should the NIH Break This Medication Patent?” https://fortune.com/2019/03/04/gilead-sciences-truvada-hiv-aids-prep-cory-johnson-act-up/
11. “HIV Prevention Drugs Illustrate Just How Bad Pharmaceutical Patents Are” https://www.nbcnews.com/think/opinion/hiv-prevention-drugs-illustrate-just-how-bad-pharmaceutical-patents-are-ncna1249428
12. “An HIV treatment cost taxpayers millions. The government patented it. But a pharma giant is making billions.” https://www.washingtonpost.com/business/economy/pharma-giant-profits-from-hiv-treatment-funded-by-taxpayers-and-patented-by-the-government/2019/03/26/cee5afb4-40fc-11e9-9361-301ffb5bd5e6_story.html
13. “NIH-Funded Study Finds Effect of PrEP on Bone Density is Reversible” https://www.niaid.nih.gov/news-events/nih-funded-study-finds-effect-prep-bone-density-reversible
14. “New research at CROI 2016: How PrEP changes kidney function” https://www.sfaf.org/collections/beta/new-research-at-croi-2016-how-prep-changes-kidney-function/
15. “An overview of tenofovir and renal disease for the HIV-treating clinician” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6111387/
16. “Tenofovir Effect on the Kidneys of HIV-Infected Patients: A Double-Edged Sword?” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3785270/
17. “New Twist in the Gilead Patent Lawsuit Over Truvada and Descovy to Prevent HIV” https://www.poz.com/article/new-twist-gilead-patent-lawsuit-truvada-descovy-prevent-hiv
18. “Truvada (emtricitabine and tenofovir disoproxil fumarate)” https://www.medicalnewstoday.com/articles/325820
19. “Truvada FDA Approval History” https://www.drugs.com/history/truvada.html
20. “What is PrEP?” https://www.plannedparenthood.org/learn/stds-hiv-safer-sex/hiv-aids/prep
21. “The Dangers of Excluding Women From HIV Prevention Drug Tests” https://www.wired.com/story/the-dangers-of-excluding-women-from-hiv-prevention-drug-tests/
22. “Lupin’s generic Truvada approved by FDA” https://www.thepharmaletter.com/in-brief/brief-lupin-s-generic-truvada-approved-by-fda
23. “Cheaper Generic PrEP Now Available in the United States” https://www.poz.com/article/cheaper-generic-prep-now-available
24. “Association of tenofovir exposure with kidney disease risk in HIV infection” https://pubmed.ncbi.nlm.nih.gov/22313955/
25. “Intentionally Delayed Pharmaceutical Innovation Under Perverse Incentives: Gilead’s HIV Pipeline As A Case Study” https://www.healthaffairs.org/do/10.1377/hblog20210614.619677/full/


