Last Updated October 26, 2020
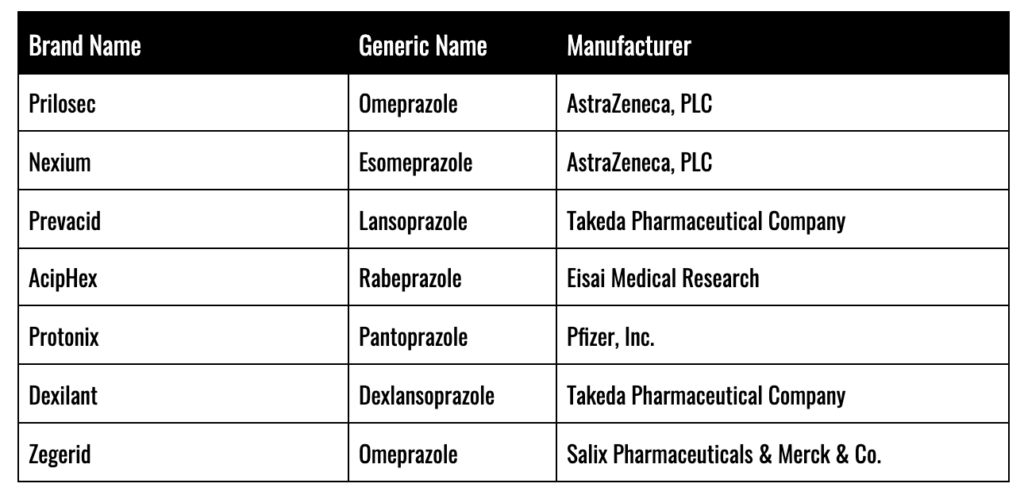
Proton Pump Inhibitors (PPIs) have been aggressively marketed and prescribed to the American public for many years as a potent tool in the fight against Gastro-Esophageal Reflux Disease (GERD) and a host of other digestive disorders. PPI drugs like Prilosec have been a runaway success for global pharmaceutical giants like AstraZeneca, PLC, accounting for over $26 billion in revenue. AstraZeneca liked Prilosec so much, in fact, that as soon as it fell out from under patent protection, it rolled out another PPI just like Prilosec, called Nexium (marketed as “The Purple Pill”) which went on to generate another $48 billion for the firm.

However, years after entering the market and after many millions of doses, clouds have emerged on the PPI horizon. Cumulative medical journal and scholarly studies published over several decades have associated long-term use of PPIs with kidney injuries. Furthermore, in 2014 the U.S. Food and Drug Administration (FDA) required prescription PPIs to carry warnings on their labels concerning Acute Interstitial Nephritis. Against this backdrop of increasingly dire scientific reporting, nearly 14,000 lawsuits alleging that PPIs have caused renal disease and related injuries have been consolidated into one massive multidistrict litigation in federal court in New Jersey (MDL-2789).
PPIs and Potential for Kidney Damage
Patients who take PPIs have demonstrated an increased risk of kidney damage that could lead to kidney failure. Researchers at the University of Buffalo School of Pharmacy have published studies showing that PPI users have a “four-fold” increase in their risk of acute kidney injuries which, in turn, can lead to more consequential injuries such as Chronic Kidney Disease.
Acute Interstitial Nephritis and Chronic Kidney Disease
PPIs have been specifically associated with a condition known as Acute Interstitial Nephritis (AIN) – a kidney disorder in which the spaces between the kidney tubules become swollen (inflamed). The risk of developing AIN from drugs such as PPIs increases over time due to prolonged use. Prolonged AIN symptoms can progress to Chronic Kidney Disease (CKD) a condition that carries with it a substantially increased risk of death and cardiovascular events.
End-Stage Renal Disease
End-Stage Renal Disease (ESRD) is typically the next stage toward complete renal failure following long-term suffering with CKD. Essentially, a sufferer’s kidneys are no longer able to function properly in a way that the body requires. Treatment for ESRD requires either frequent dialysis or a kidney transplant for the patient to survive.
Lawsuits and Allegations
Complaints have been filed in courts across the United States, many of which have been consolidated in MDL-2789, by people suffering from a range of renal maladies including AIN, CKD, and ESRD who also took either prescription or over-the-counter (OTC) versions of PPIs (or both). In their complaints, many sufferers allege that manufacturers knew of the risks of renal damage from PPIs (in particular Nexium) going back to the late 1980s and that research as far back as 1992 associated PPIs with kidney damage. Furthermore, they claim, studies by the Yale School of Medicine in 2006 made findings of a connection between PPIs, AIN, and CKD in most patients. Some of the claims also reference a 2016 study in the Journal of the American Society of Nephrology that found that PPI use, including Nexium, was independently associated with 20%-to-50% higher risk for CKD.
Sufferers allege that, with all of the mounting scientific evidence compiling, that companies like AstraZeneca and other manufacturers either knew or should have known of the increased risk for CKD posed by PPIs. They claim that manufacturers instead took no action to warn doctors or patients of the risk of kidney disease – and even continued to claim that PPIs such as Nexium did not have any associated risks of kidney injury.
Lawsuit Profiles
Hope Butler
A resident of Ohio, Ms. Butler was among the very first to file a PPI lawsuit naming AstraZeneca in the U.S. District Court for the Southern District of Ohio in March 2017. She states in her complaint that she used prescription Nexium between 2010 and December 2016. Ms. Butler filed her lawsuit after her diagnosis with CKD in March 2015 and her discovery of the increased potential for kidney disease associated with PPIs.
Cheryl Lear
Cheryl Lear, a chronic sufferer with GERD, nonsteroidal anti-inflammatory drug-induced gastropathy, and other peptic disorders from Duval County, Florida, filed a lawsuit naming AstraZeneca, Wyeth Pharmaceuticals, and Pfizer, Inc. (among others) in federal court in 2017. She began taking prescription Prilosec in 2012 and later prescription Protonix through September 2013. Ms. Lear filed her lawsuit after suffering several years with acute kidney injuries, culminating with CKD, and upon learning of the potential connection with her long term use of Prilosec and Protonix
Lawsuits Are Still Being Filed
Individuals who have taken either a prescription or OTC PPIs and have suffered from kidney damage or related illnesses may be able to take legal action. Although each potential case is unique, types of compensation sought typically include medical expenses, loss of income, and pain and suffering. Case reviews are free and do not involve any obligation whatsoever.
Sources Cited (37)
1. “In re: Proton Pumps Inhibitor Litigation (MDL-2789)” https://ecf.jpml.uscourts.gov/doc1/8501834105
2. “Hope Butler v. AstraZeneca Pharmaceuticals, LP, et al.” https://ecf.ohsd.uscourts.gov/doc1/14316373880
3. “Cheryl Lear v. AstraZeneca Pharmaceuticals, LP. et al.” https://ecf.flmd.uscourts.gov/doc1/047117162494
4. “Kidney Damage Tied to Proton Pump Inhibitors” https://www.renalandurologynews.com/home/news/nephrology/acute-kidney-injury/kidney-damage-tied-to-proton-pump-inhibitors/#:~:text=A%20large%20retrospective%20study%20found,a%20large%20population%2Dbased%20study.
5. “Acute interstitial nephritis due to proton pump inhibitors” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3741979/
6. “Proton Pump Inhibitors and CKD” https://jasn.asnjournals.org/content/27/10/2926
7. “Popular heartburn drugs linked to gradual yet ‘silent’ kidney damage” https://medicine.wustl.edu/news/popular-heartburn-drugs-linked-gradual-yet-silent-kidney-damage/
8. “Proton Pump Inhibitor Use and Risk of Chronic Kidney Disease” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4772730/
9. “Chronic PPI Use Linked to Declining Kidney Function in Chronic Kidney Disease” https://www.pharmacytimes.com/news/chronic-ppi-use-linked-to-declining-kidney-function-in-chronic-kidney-disease
10. “Diagnosis and Management of Acute Interstitial Nephritis” https://www.aafp.org/afp/2003/0615/p2527.html#:~:text=Acute%20interstitial%20nephritis%20(AIN)%20defines,edema%20of%20the%20renal%20interstitium.
11. “Interstitial nephritis” https://medlineplus.gov/ency/article/000464.htm
12. “PPIs and kidney disease: from AIN to CKD” https://pubmed.ncbi.nlm.nih.gov/27072818/
13. “End Stage Renal Disease” https://pubmed.ncbi.nlm.nih.gov/27072818/
14. “The physiological background behind and course of development of the first proton pump inhibitor” https://pubmed.ncbi.nlm.nih.gov/25857639/
15. “Harvard Health Letter: Proton-pump inhibitors” https://www.health.harvard.edu/diseases-and-conditions/proton-pump-inhibitors
16. “MedlinePlus: Proton Pump Inhibitors” https://medlineplus.gov/ency/patientinstructions/000381.htm
17. “Healthline: Proton Pump Inhibitors” https://www.healthline.com/health/gerd/proton-pump-inhibitors#1
18. “The Safety of Appropriate Use of Over-the-Counter Proton Pump Inhibitors: An Evidence-Based Review and Delphi Consensus” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5357248/
19. “What you should know about: PPIs” https://www.health.harvard.edu/diseases-and-conditions/what-you-should-know-about-ppis#:~:text=PPIs%20work%20by%20inhibiting%20certain,over%20a%20period%20of%20time.
20. “Proton Pump Inhibitors: Use in Adults” https://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Pharmacy-Education-Materials/Downloads/ppi-adult-factsheet11-14.pdf
21. “25 Years of Proton Pump Inhibitors: A Comprehensive Review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5221858/
22. “Mayo Clinic: Omeprazole (Oral Route)” https://www.mayoclinic.org/drugs-supplements/omeprazole-oral-route/description/drg-20066836
23. “Mayo Clinic Proceedings: Proton Pump Inhibitors: Review of Emerging Concerns” https://www.mayoclinicproceedings.org/article/S0025-6196(17)30841-8/fulltext#:~:text=Although%20PPIs%20have%20had%20an,chronic%20kidney%20disease%2C%20and%20dementia.
24. “The risks of long-term use of proton pump inhibitors: a critical review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6463334/
25. “Advantages and Disadvantages of Long-term Proton Pump Inhibitor Use” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5885718/
26. “Pharmacokinetic Drug Interaction Profiles of Proton Pump Inhibitors: An Update” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3975086/
27. “Drug-drug interaction profiles of proton pump inhibitors” https://pubmed.ncbi.nlm.nih.gov/20608754/
28. “Proton Pump Inhibitors: Considerations With Long-Term Use” https://www.uspharmacist.com/article/proton-pump-inhibitors-considerations-with-longterm-use
29. “FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors” https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-possible-increased-risk-fractures-hip-wrist-and-spine-use-proton-pump#:~:text=FDA%20has%20determined%20an%20osteoporosis,dose%20PPI%20use%20is%20unlikely.
30. “FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs)” https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-low-magnesium-levels-can-be-associated-long-term-use-proton-pump
31. “Forbes Magazine: Hard to Swallow” https://www.forbes.com/forbes/2002/0513/199.html#3a838e544282
32. “American Council on Science and Health: Nexium: The Dark Side Of Pharma” https://www.acsh.org/news/2017/01/18/nexium-dark-side-pharma-10546
33. “Is the concomitant use of clopidogrel and Proton Pump Inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: a systematic review and meta-analysis of recently published studies (2012 – 2016)” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5221663/#:~:text=Because%20PPIs%20can%20act%20as,increase%20in%20adverse%20clinical%20outcomes.
34. “Harvard Medical School – Trends in Medicine: The Link Between Proton Pump Inhibitors and Kidney Disease” https://trends.hms.harvard.edu/2017/04/27/the-link-between-proton-pump-inhibitors-and-kidney-disease/#:~:text=In%20a%20recent%20meta%2Danalysis,1.07%2D1.72%2C%20respectively).
35. “Association of Long-term Proton Pump Inhibitor Therapy with Bone Fractures and effects on Absorption of Calcium, Vitamin B12, Iron, and Magnesium” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2974811/
36. “Harvard Women’s Health Watch: By the way, doctor: Does long-term use of Prilosec cause stomach cancer?” https://www.health.harvard.edu/diseases-and-conditions/by_the_way_doctor_does_long-term_use_of_prilosec_cause_stomach_cancer#:~:text=This%20has%20raised%20some%20concerns,increased%20risk%20for%20stomach%20cancer.
37. “FDA Warns About Bone Breaks And Heartburn Drugs” https://www.npr.org/sections/health-shots/2010/05/26/127132723/fda-warns-about-bone-risks-from-heartburn-drugs