Updated November 21, 2020
Zantac is the brand name of ranitidine, one of the most popular medications ever used to treat and prevent conditions such as heartburn; ulcers, and Gastro-Esophageal Reflux Disease (GERD). It is part of a family of drugs known as H2-blockers for their method of action blocking histamine cells in the stomach.
Zantac was one of the best-selling drugs in history and made enormous profits for both GlaxoSmithKline and Sanofi as well as serving as a center of enormous prestige for both companies. However, those profits and prestige took a big hit in 2019 when a small testing lab in Connecticut discovered trace amounts of N-nitrosodimethylamine (NDMA) in batches of ranitidine. NDMA has been recognized by the U.S. Environmental Protection Agency (EPA) and the International Agency for Research on Cancer (IARC) as a probable human carcinogen.
Upon the release of these results, some generic manufacturers of ranitidine began voluntary recalls of their products in 2019. This was followed by the announcement of a mandatory withdrawal of ranitidine from the market by the U.S. Food and Drug Administration (FDA) on April 1, 2020.
What is NDMA?
According to the EPA, NDMA is a semivolatile organic chemical. Although it is not typically produced intentionally in the United States, it can be produced unintentionally through chemical reactions in products or industrial processes. It is believed that when NDMA is consumed, it modifies cellular DNA, increasing cancer risk substantially. Studies of NDMA and cancer risk have largely taken place only in animals as direct research from humans is still very limited.
How Did NDMA Wind Up in Zantac?
Valisure, a small laboratory in Connecticut, was the first testing facility to discover and report on NDMA contamination in Zantac/ranitidine. The lab believes that the NDMA in ranitidine is the result of the molecular instability of the drug itself and may form as a natural byproduct of its breakdown over time.
What Cancers are Associated with NDMA?
Cancer risk can stem from a number of factors unique to each person and their circumstances. Cancer can be linked to things such as infectious agents; genetic predisposition; behavioral factors and habits like diet, smoking, alcohol use, obesity, and diet; or even exposure to pollution. Also, it is important to remember that researchers have not yet established a conclusive link between Zantac NDMA contamination and cancer. However, it is theorized that long-term exposure to NDMA could be linked with the following cancers:
- Stomach
- Bladder
- Colorectal
- Liver
- Esophageal
- Small Intestinal
Although less prevalent in research, NDMA has also been linked to these types of cancers:
- Breast
- Thyroid
- Testicular
- Leukemia
- Non-Hodgkin’s Lymphoma
- Multiple Myeloma
- Prostate
- Ovarian
- Pancreatic
- Lung
- Uterine
- Brain
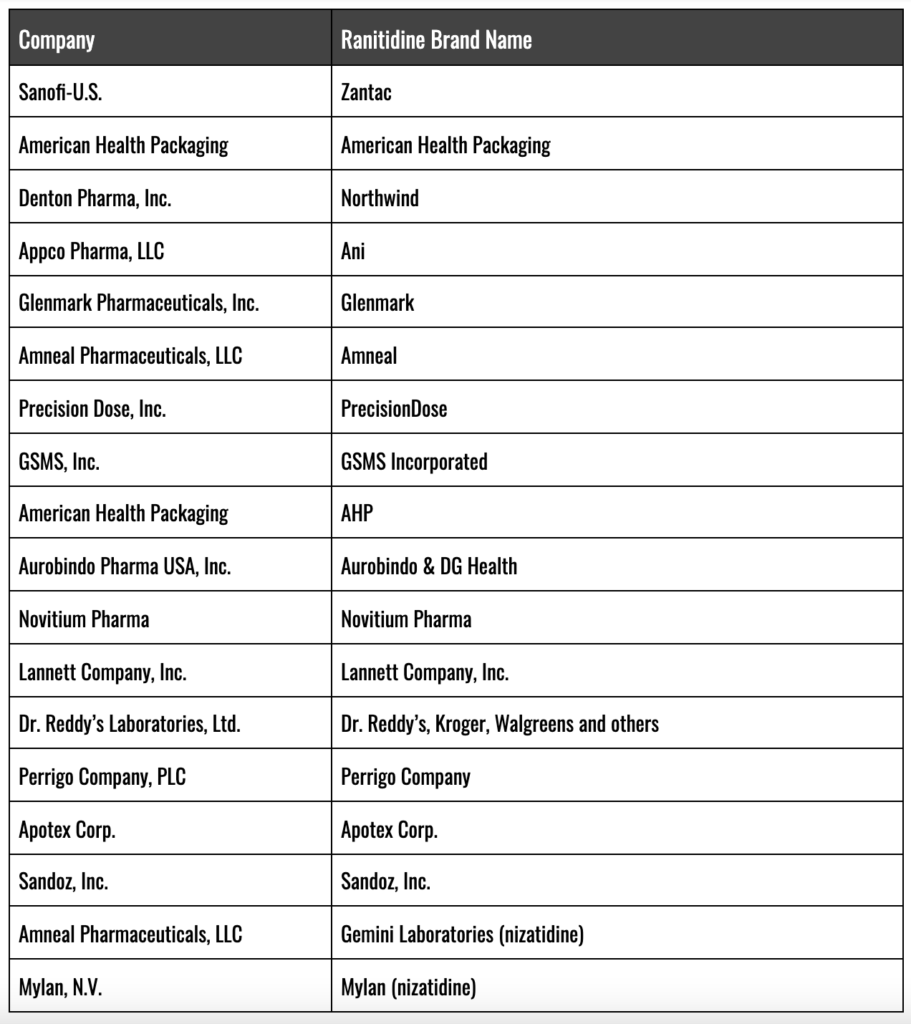
Ranitidine Manufacturers and FDA Recall
Upon announcement of the Valisure laboratory results, several generic manufacturers of ranitidine, led by Sandoz, both halted the distribution of their ranitidine products and later recalled their brands from store shelves. However, Sanofi, the manufacturer of branded Zantac ranitidine tablets initially declined to halt sales of Zantac. The company instead publicly stated that “The FDA reported levels of [NDMA] in ranitidine in preliminary tests barely exceed amounts found in common foods”.
On April 1, 2020, the FDA announced a complete market withdrawal and removal from shelves of all prescription and over-the-counter (OTC) forms of Zantac and generic equivalents of ranitidine. The agency said its decision was prompted by Valisure’s testing as well as its own testing which revealed the presence of NDMA under normal storage conditions, and even higher amounts when ranitidine is stored at higher temperatures. Furthermore, the FDA’s testing revealed that the older a ranitidine product is (the length of time following manufacture) the greater the amount of NDMA is present.
Branded Zantac and Generic Ranitidine Brands Recalled/Withdrawn
Sources Cited (31):
1) “Popular heartburn drug ranitidine recalled: What you need to know and do” https://www.health.harvard.edu/blog/popular-heartburn-drug-ranitidine-recalled-what-you-need-to-know-and-do-2019092817911
2) “Technical Fact Sheet – N-Nitroso-dimethylamine (NDMA)” https://www.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_contaminant_ndma_january2014_final.pdf
3) “The Zantac problem: What’s NDMA?” https://abcnews.go.com/Health/zantac-problem-whats-ndma/story?id=65799147
4) “FDA Requests Removal of All Ranitidine Products (Zantac) from the Market” https://www.fda.gov/news-events/press-announcements/fda-requests-removal-all-ranitidine-products-zantac-market
5) “FDA Updates and Press Announcements on NDMA in Zantac (ranitidine)” https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-ndma-zantac-ranitidine
6) “Recalls, Market Withdrawals, & Safety Alerts” https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts
7) “NDMA, a contaminant found in multiple drugs, has industry seeking sources and solutions” https://cen.acs.org/pharmaceuticals/pharmaceutical-chemicals/NDMA-contaminant-found-multiple-drugs/98/i15
8) “Ranitidine Cancer Risk“ https://www.medpagetoday.com/meetingcoverage/ddw/86314
9) “Health Risks Associated with N-Nitrosodimethylamine (NDMA)” https://www.labmanager.com/insights/health-risks-associated-with-n-nitrosodimethylamine-ndma-656
10) “What We Know about the Possible Carcinogen Found in Zantac” https://www.scientificamerican.com/article/what-we-know-about-the-possible-carcinogen-found-in-zantac/#:~:text=In%20its%20petition%2C%20Valisure%20also,break%20down%20to%20form%20NDMA.
11) “The Finding of N‐Nitrosodimethylamine in Common Medicines” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7288647/
12) “Oral intake of ranitidine increases urinary excretion of N-nitrosodimethylamine” https://pubmed.ncbi.nlm.nih.gov/26992900/
13) “Formation Mechanism of NDMA from Ranitidine, Trimethylamine, and Other Tertiary Amines during Chloramination: A Computational Study” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4123930/
14) “Clinical Study to Investigate the Urinary Excretion of N-nitrosodimethylamine (NDMA) After Ranitidine Administration” https://clinicaltrials.gov/ct2/show/NCT04397445
15) “FDA asks manufacturers to remove ranitidine from market: What now?” https://www.aappublications.org/news/2020/07/01/focus-ranitidine070120
16) “Concerned about the ranitidine (brand name: Zantac) recall? Here’s what the FDA says” https://www.miamiherald.com/news/health-care/article236067123.html
17) “Some ranitidine (Zantac) has tested safe, but more recalled for too much carcinogen” https://www.miamiherald.com/news/health-care/article237219936.html
18) “A tiny pharmacy is identifying big problems with common drugs, including Zantac” https://www.washingtonpost.com/science/a-tiny-pharmacy-is-identifying-big-problems-with-common-drugs-including-zantac/2019/11/08/6dd009ca-eb76-11e9-9c6d-436a0df4f31d_story.html
19) “N-Nitrosodimethylamine (NDMA) as a Drinking Water Contaminant: A Review” https://superfund.berkeley.edu/pdf/231.pdf
20) “Questions and Answers: NDMA impurities in ranitidine (commonly known as Zantac)” https://www.fda.gov/drugs/drug-safety-and-availability/questions-and-answers-ndma-impurities-ranitidine-commonly-known-zantac#:~:text=FDA%20has%20found%20N%2Dnitrosodimethylamine,ranitidine%20they%20may%20currently%20have.
21) “Our Mission” https://www.valisure.com/about-us/
22) “Formation Mechanism of NDMA from Ranitidine, Trimethylamine, and Other Tertiary Amines during Chloramination: A Computational Study” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4123930/
23) “The Finding of N‐Nitrosodimethylamine in Common Medicines” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7288647/
24) “Oral intake of ranitidine increases urinary excretion of N-nitrosodimethylamine” https://pubmed.ncbi.nlm.nih.gov/26992900/
25) “Zantac, Ranitidine Pulled From Market, Here Are FDA’s Latest Cancer Concerns” https://www.forbes.com/sites/brucelee/2020/04/02/zantac-ranitidine-pulled-from-market-here-are-fdas-latest-cancer-concerns/#e60d6fb2b8c5
26) “Zantac is prescribed 15 million times a year. So how did it become a potential cancer risk?” https://www.usatoday.com/story/news/health/2019/11/07/how-did-zantac-become-potential-cancer-risk-fda-wants-find-out/2509043001/
27) “Ranitidine (Zantac) recall expanded, many questions remain” https://www.health.harvard.edu/blog/ranitidine-zantac-recall-expanded-many-questions-remain-2020040218044
28) “Lab finds NDMA in Zantac can develop during storage” https://www.fiercepharma.com/manufacturing/lab-finds-ndma-zantac-can-develop-during-storage-bloomberg
29) “FDA Recalls All Ranitidine (Zantac) Products, Citing Increased Risk of Cancer” https://www.ajmc.com/view/fda-recalls-all-ranitidine-products-zantac-citing-increased-risk-of-cancer
30) “Valisure Detects NDMA in Ranitidine” https://www.valisure.com/blog/valisure-news/detection-of-ndma-in-raniditine/
31) “COVID-19 and Beyond: Oversight of the FDA’s Foreign Drug Manufacturing Inspection Process – Hearing before the United States Senate Committee on Finance (June 2, 2020)” https://www.finance.senate.gov/imo/media/doc/02JUN2020.VALISURE.LIGHT.STMNT.pdf