
The U.S. Food and Drug Administration (FDA) has primary responsibility for regulating what must be included on the labels, including Black Box warnings, for prescription pharmaceutical medications and biological products. What is included on the label derives primarily from disclosures to the FDA by drug manufacturers and are submitted as part of the approval process. Although the FDA drug approval process imposes many requirements on manufacturers in the drug “pre-approval” stage, these companies continue to have responsibility for monitoring the ongoing safety and efficacy of their products in “post-market”.
After approval, the FDA may require drug manufacturers to include additional new information on drug labels, such as boxed warnings which advise doctors and pharmacists that a particular drug may cause serious adverse reactions or other problems. These are known as “black box warnings” and are reserved for those drugs which have demonstrated a potential for side effects which can lead to death, disability or serious injury.
What Is the “Black Box” Warning?
The black box is the most substantial and serious of the on-label warnings that the FDA issues for drugs, while still allowing them to remain on the market. The name “black box” comes from the black-lined border around the text of the warning. Black box warnings must appear on the label of the prescription in order to alert physicians, pharmacists and consumers about safety concerns, serious side effects or risks to life. The information in a black box warning must provide a concise summary of the adverse effects and risks associated with the drug.
Here is an example of an FDA mandated black box warning on a label for Fluoroquinolone Antibiotics:
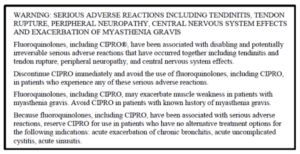
How Well Do Physicians and Pharmacists Understand the Warnings?

Although the black box advises consumers about serious side effects and risks, its primary purpose is to advise doctors and pharmacists as “learned intermediaries” and elevate the information to their attention. The FDA itself notes that black boxes are “…designed to make information in prescription drug labeling easier for healthcare practitioners to access, read and use to make prescribing decisions.”
Doctors are trained to be aware of the presence of black box warnings and as a general rule, always want their patients to have as much informed consent about their prescriptions as is possible. Many physicians have affirmed that black box warnings do affect their prescribing and treatment decisions – with some admitting to a hesitancy to prescribe anything with a black box warning unless no other treatment options or therapies are available.
Unfortunately, reports over the years suggest that the influence of black box warnings on physicians is not uniform owing to either a lack of understanding about the meaning of the black box or just simply being unaware of the warning itself. In a Harvard Medical School study of 930,000 ambulatory care patients, it was learned that 42% of patients had received prescriptions for black box labeled medications and that corresponding physician compliance with the recommendations on the label were “variable” in nature.
Furthermore, non-profit foundations and agencies which monitor the FDA have expressed concerns about the effectiveness of black box warnings. The sheer volume of post-market warnings issued by the FDA is leading some physicians to complain of “data fatigue” as the cause for overlooking drug warnings. For example: in 2004, the FDA issued 68 safety alerts for market recalls for drugs and devices. In 2005, that number shot up to 127. Accordingly, some experts have even gone so far as to claim that the FDA is losing its ability to influence the practice of medicine and prescribing of drugs, brandishing estimates that as many as 80% of safety warnings are overlooked by physicians and pharmacists.
The FDA “Fast Track” Process
If physicians and pharmacists are overwhelmed by warning data coming from the FDA, is it possible that too many dangerous drugs are making it through the approval process without further study? Some experts think so.
In 1988, the FDA introduced its first “fast track” drug approval process to facilitate the “development and expedite the review of drugs to treat serious illnesses and fill unmet medical need”. In essence, fast track was created to shorten the approval process for serious or rare diseases, especially those without other available treatments. However, in 1992 fast track was augmented by the FDA with two additional avenues for approval: “priority review” and “accelerated approval”.
Since 1992 and the enactment of these additional expedited aveues, median approval times for new drugs decreased from 33.6 months to 16.1 months. Accordingly, some experts and their counterparts in the drug manufacturing industry point to this fact and argue that the fast track process provides benefits for society-at-large with more medications getting onto the market in shorter time. However, others argue that it has also undermined safety fundamentally. They note that between 1999 and 2009, outpatient prescribers in the United States wrote more than 30 million prescriptions for each of nine drugs (for a total of 270 million prescriptions) that were subsequently removed from the market for safety reasons or which received black box warnings for potentially lethal side effects.
One statistical study, researched over several years and presented for review in 2014, concluded that newly approved drugs have a one-in-three chance of acquiring a new black box warning or being withdrawn for safety reasons within 25 years of FDA approval. This study made the recommendation that the FDA: a) strengthen drug approval standards (i.e. increase approval times); and b) that physicians should rely on drugs with longer market exposure and established track records – unless it involves a truly breakthrough therapy.
Future of Black Box Warnings
For too many physicians, the black box warnings are opaque in their meaning and lack the reasoning that seasoned medical professionals utilize when evaluating the needs of their patients. Furthermore, the FDA relies upon a system of post market “surveillance” for learning about adverse effects, that is itself wholly reliant upon the drug manufacturer collecting and reporting adverse data to the FDA. For these reasons, many both in the consumer safety and drug regulatory fields feel that the FDA should: a) improve and strengthen the methods it uses to communicate adverse side effects to physicians and pharmacists; b) increase scrutiny and approval times for drugs in the pipeline; and c) enhance post-market surveillance with less reliance on reporting by the manufacturers themselves.
Sources Cited (14)
1) “A Guide to Drug Safety Terms at FDA” https://www.fda.gov/media/74382/download
2) “What Does it Mean if My Medication Has a ‘Black Box’ Warning?” https://health.clevelandclinic.org/what-does-it-mean-if-my-medication-has-a-black-box-warning/
3) “New and Incremental FDA Black Box Warnings From 2008 to 2015” https://pubmed.ncbi.nlm.nih.gov/29215916/
4) “Medicare Prescription Drug Plan Formulary Restrictions After Postmarket FDA Black Box Warnings” https://pubmed.ncbi.nlm.nih.gov/31663461/
5) “The meaning behind black box and other drug warnings” https://www.healio.com/pediatrics/practice-management/news/print/infectious-diseases-in-children/%7B2f3d205a-694f-4ff0-9d60-56563e17f149%7D/the-meaning-behind-black-box-and-other-drug-warnings
6) “Black box” 101: How the Food and Drug Administration evaluates, communicates, and manages drug benefit/risk” https://www.jacionline.org/article/S0091-6749(05)02325-0/fulltext
7) “Expedited drug review process: Fast, but flawed” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4936080/
8) “Era Of Faster FDA Drug Approval Has Also Seen Increased Black-Box Warnings And Market Withdrawals” https://www.healthaffairs.org/doi/10.1377/hlthaff.2014.0122
9) “Why You Should Pay Attention to Black Box Warnings on Medication” https://www.verywellhealth.com/black-box-warnings-1124107#what-does-one-look-like
10) “Physicians May Overlook Black Box Warnings” https://psychnews.psychiatryonline.org/doi/pdf/10.1176/pn.41.6.0013
11) “Consumers Tune Out FDA Warnings” https://money.cnn.com/2008/02/22/news/companies/fdawarning_fatigue/index.htm
12) “The consent risks with ‘black box’ warning medication” https://www.hrmronline.com/article/the-consent-risks-with-black-box-warning-medication
13) “What is a Black Box Warning for a Drug?” https://www.everydayhealth.com/fda/what-black-box-warning-drug/
14) “Doctors often ignore ‘black box’ warnings on drugs” https://www.reliasmedia.com/articles/120625-doctors-often-ignore-black-box-warnings-on-drugs
