Last Updated September 28, 2020
An Inferior Vena Cava (IVC) filter is a small mechanical device placed into one of the largest vascular pathways in the human body to prevent clots from circulating from the lower limbs into the lungs causing a Pulmonary Embolism (PE). IVC filters are not necessarily “new” technology. They were first cleared for use by the U.S. Food and Drug Administration (FDA) through the abbreviated 510(k) Clearance process in the late 1970s. However, their use expanded dramatically in a very short span of time between 1999 and 2008.

In 2010, the FDA, after noticing a spike of “adverse event reports” through the previous years, issued a warning about IVC filters and the potential for such issues as filter migration; embolization; perforation of the Vena Cava; and mechanical fracturing of the filter itself. The FDA’s warning was followed in 2014 with a “safety communication” concerning the filters and the agency instituted a new postmarket surveillance study to evaluate the growing use of IVC filters and to make additional recommendations.
As of late 2020, nearly 15,000 lawsuits naming IVC manufacturers have been filed in multiple states, with the largest number of parties filing against manufacturers C.R. Bard and Cook Medical for their devices. These lawsuits have been consolidated into multidistrict litigation (MDL) in Arizona and Indiana.
Alleged Issues with IVC Filters
In recent lawsuits, patients implanted with IVC filters manufactured by Cook Medical and C.R. Bard alleged that due to their use of the 510(k) Clearance process, the companies failed to conduct any clinical testing or animal studies to determine how these filters would function once they are permanently implanted in the human body. Furthermore, the implantees alleged that Cook Medical and C.R. Bard knew or should have known that these devices are defective and dangerous; and that they have a high propensity for:
- Fracture
- Migration
- Excessive Tilting
- Perforation of the Vena Cava
Finally, the patients alleged that the companies were aware of defects with their filters in the face of increasing adverse reporting to the FDA. Nonetheless, they continued to aggressively market the devices while misrepresenting that they had much a better safety record than what was actually reported.
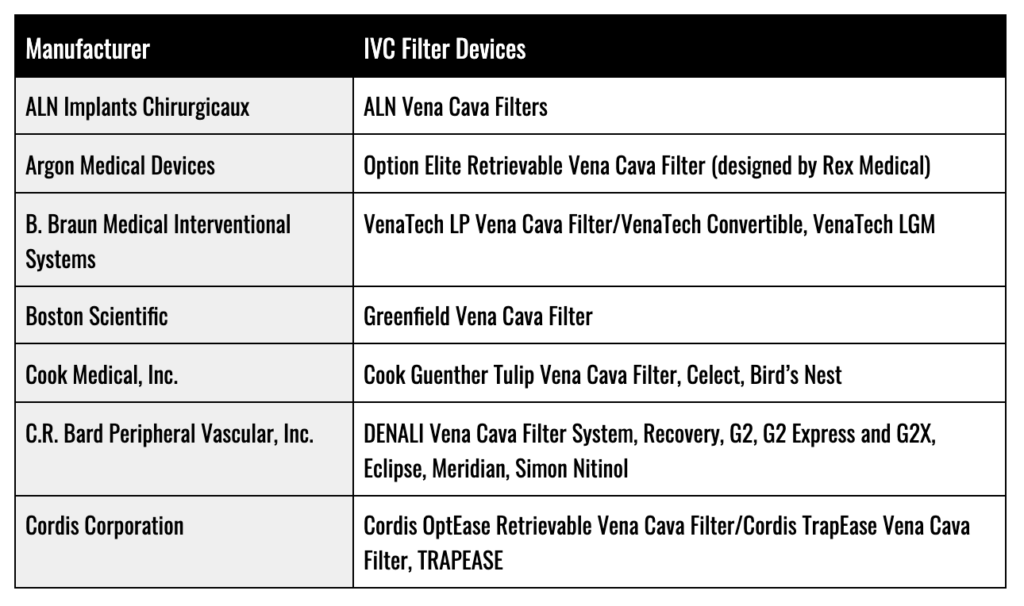
IVC Filter Manufacturers
FDA Recalls and Administrative Action on IVC Filters
To date, the FDA has yet to issue any formal recalls for an IVC filter device. Nonetheless, between 2005 and 2019, at least eight types of IVF filters have been voluntarily withdrawn from the market by their manufacturers. In its first warning letter concerning IVC filters in 2010, the FDA detailed over 921 injuries from IVC filters spanning: migration through the body; migration into the heart and lungs (embolization); perforation of the vena cava; and filter fracturing. Since 2014, filter manufacturers have been given the option of either participating in the FDA’s 522 Postmarket Surveillance Studies program or an independent clinical study known as “Predicting the Safety and Effectiveness of Inferior Vena Cava Filters” (PRESERVE).
IVC Filter Lawsuits
In re: Bard IVC Filters (MDL-2641)
Lawsuits involving C.R. Bard’s Recovery and G2 IVC filter lines began emerging in late 2013 and early 2014. Due to the number and similarity of the allegations leveled in the complaints, they were consolidated into multidistrict litigation in Arizona. In their complaints, the implantees alleged that manufacturing and design defects in the Recovery and G2 lines, which they claimed C.R. Bard knew all along, were causing them to experience significant rates of fracture and migration. Some studies cited in the complaints stated that the Recovery line suffered from a 21% to 31.7% rate of fracture. Beyond the manufacturing and design defect claims, implantees also alleged that C.R. Bard actively denied and concealed knowledge of the dangers posed by the Recovery and G2 IVC filters and withheld such evidence in an attempt to delay court filings.
In March 2018, at the conclusion of the first bellwether trial for this MDL, a jury awarded plaintiff, Sherr-Una Booker $3.6 million in compensatory and punitive damages. Thereafter, C.R. Bard prevailed in two bellwether trials in June 2018 and October 2018. In May 2019, the judge presiding over this MDL announced a limited settlement for thousands of cases with the rest sent back to continue proceedings in state courts across the country. The MDL itself is now closed out, however, cases may still be brought in state court.
In re: Cook Medical, Inc. IVC Filters (MDL-2570)
Similar to the MDL involving C.R. Bard, cases revolving around the Gunther Tulip and Celect lines of IVC filters manufactured by Cook Medical, Inc., began to make their way into courtrooms around the country in 2013 and 2014. These cases were then consolidated into multidistrict litigation in Indiana. Like the plaintiffs in the C.R. Bard cases, the implantees in this MDL complained of alleged design and manufacturing defects such as tilt, migration, and fracturing. Specifically, some claimants referenced a study published in Cardiovascular Interventional Radiology in 2012 which assessed that the Gunther Tulip and Celect lines failed at a rate of 100% up to 71 days following implant and caused some degree of perforation of the wall of the vena cava. The same study reported that tilt was witnessed in 40% of the Gunther Tulip and Celect filter lines.
The outcomes of the various bellwether trials in this MDL have been a mixed-bag:
- In November 2017, a jury sided with Cook Medical and failed to find the company liable.
- A second bellwether case was dismissed in April 2018 due to the statute of limitation issues.
- A Houston firefighter was awarded $1.2 million by a Texas jury in May 2018 (not actually a bellwether case and not part of the MDL).
- In December 2018, the presiding judge in the MDL granted summary judgment to Cook Medical in a Georgia case brought by Tonya Brand.
- Most recently, in February 2019, an Indiana jury awarded $3 million to a woman who suffered a range of injuries from one of its IVC filter lines.
Sources Cited (20)
1. “Long-Term Clinical Outcomes of Complicated Retrievable Inferior Vena Cava Filter for Deep Venous Thrombosis Patients: Safety and Effectiveness” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6330022/
2. “Inferior Vena Cava Filter Placement and Removal” https://www.radiologyinfo.org/en/info.cfm?pg=venacavafilter
3. “Filter tilting and retrievability of the Celect and Denali inferior vena cava filters using propensity score-matching analysis” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6134324/
4. “About Your Inferior Vena Cava (IVC) Filter Placement” https://www.mskcc.org/cancer-care/patient-education/ivc-filter-placement
5. “What is the evidence behind the IVC filter?” https://emcrit.org/pulmcrit/what-is-the-evidence-behind-the-ivc-filter/#:~:text=37%20patients%20with%20recurrent%20venous,rate%20of%20IVC%20filter%20thrombosis.&text=Therefore%2C%20they%20concluded%20that%20this,two%20problems%20with%20this%20argument.
6. “Vena Cava Filters” https://my.clevelandclinic.org/health/treatments/17609-vena-cava-filters
7. “Vena cava filters: Tiny cages that trap blood clots” https://www.health.harvard.edu/heart-health/vena-cava-filters-tiny-cages-that-trap-blood-clots
8. “Inferior Vena Cava Filters: Guidelines, Best Practice, and Expanding Indications” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862857/#:~:text=There%20are%20two%20general%20types,and%20absolute%20contraindications%20to%20anticoagulation.
9. “Inferior Vena Cava Filter” https://www.ncbi.nlm.nih.gov/books/NBK549900/
10. “Permanent versus Retrievable Inferior Vena Cava Filters: Rethinking the “One-Filter-for-All” Approach to Mechanical Thromboembolic Prophylaxis” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862850/
11. “FDA Issues Statement on Treatment and Follow-Up Regarding IVC Filter Complications” https://evtoday.com/news/fda-issues-statement-on-treatment-and-follow-up-regarding-ivc-filter-complications
12. “Predicting the Safety and Effectiveness of Inferior Vena Cava Filters” http://www.preservetrial.com/
13. “Vena cava filters” https://www.medicalexpo.com/medical-manufacturer/vena-cava-filter-44592.html
14. “Complications of Inferior Vena Caval Filters” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3036364/
15. “IVC Filter Migration: How Does It Happen?” https://medium.com/@Drug_Justice/ivc-filter-migration-how-does-it-happen-1e453d77a0af
16. “FDA Updates Safety Communication on IVC Filter Retrieval” https://evtoday.com/news/fda-updates-safety-communication-on-ivc-filter-retrieval
17. “Lara L. Adams et al. v. Cook Medical Incorporated, et al” https://www.courtlistener.com/docket/5117387/adams-v-cook-medical/
18. “Charles Conn et al. v. C.R. Bard et al.” https://www.pacermonitor.com/public/case/9216987/Conn_et_al_v_C_R_Bard_Incorporated_et_al
19. “Perforation of the IVC: rule rather than exception after longer indwelling times for the Günther Tulip and Celect retrievable filters” https://pubmed.ncbi.nlm.nih.gov/21448771/
20. “Complications of Celect, Günther Tulip, and Greenfield Inferior Vena Cava Filters on CT Follow-up: A Single-Institution Experience” https://www.jvir.org/article/S1051-0443(13)01258-X/abstract


