Last Updated November 14, 2020
Hernia repair is one of the most common surgical procedures performed in the United States and it is estimated that over 20 million of them occur globally each year. Surgical meshes have been the preferred repair format by the medical profession for over 100 years and more than 80% of hernia repairs in the U.S. use mesh products. Accordingly, a great deal of research over the decades has been focused on developing new meshes made from both organic as well as synthetic materials.
Researchers and surgeons have examined the use of metals, polymers, and biodegradable materials for years as the basis for mesh that is simultaneously resistant to infection will maintain tensile strength, and will rapidly assimilate/incorporate into the surrounding tissue. In the 1950s, Dr. Francis Usher first examined the use of materials such as Nylon, Orlon, Dacron, and Teflon as possible mesh candidate materials. Usher eventually settled on a material known as Marlex and within two years introduced the first marlex-based woven hernia mesh.
On the basis of his work with Marlex, Dr. Usher later discovered that polypropylene mesh offered even more advantages due to its rapid incorporation into surrounding tissue and ease of sterilization. By 1998, research into polypropylene mesh materials had advanced enough that next-generation “lightweight” polypropylene hernia meshes were introduced into the market, largely through the U.S. Food and Drug Administration’s (FDA) “fast track” 510(k) clearance process.
Today there are more than 70 types of mesh on the market. However, despite their prevalence in hernia repair procedures, meshes – and in particular, ones made from polypropylene, have not all proven to be a huge benefit for patients. Some polypropylene mesh products have been linked with serious side effects that include severe pain, infections, tissue fusion, organ perforation, and worse. At the moment, three mesh manufacturers: Ethicon (Johnson & Johnson); Atrium Medical, and Davol (C.R. Bard) are embroiled in large-scale multidistrict litigation (MDL) concerning some of their best-selling hernia mesh products.
Polypropylene Basics
Polypropylene is a “thermoplastic” meaning that at high temperatures its form can be easily manipulated, extruded, or molded – later solidifying when it cools down. It is used in a variety of situations and consumer products ranging from essential parts used in the automotive industry to clothing and even in the surgical mesh.
Polypropylene is typically manufactured either through injection molding or using modern printing/cutting devices called “CNC” machines (Computer Numerical Control). It is preferred for its properties in the surgical setting which include: greater elasticity and toughness; as well as resistance to fatigue. Additionally, polypropylene is relatively inexpensive – making it even more desirable in the highly competitive and lucrative hernia mesh market.

Use of Polypropylene Mesh in Hernia Repair Procedures
Generally, surgical mesh is used to repair hernias in either a laparoscopic or open repair setting. In a laparoscopic procedure, a surgeon will make small incisions into the abdomen with surgical tools that can both repair the hernia and insert/set the mesh device. Open hernia repairs are more invasive and involve a larger incision in the abdomen to either suture the hernia or place a surgical mesh.
Polypropylene mesh is considered to be a “permanent” mesh material meaning it is non-absorbable. Permanent materials like polypropylene have virtues in their strength and ability to be easily manipulated. The size of the pores in polypropylene mesh can have a substantial impact on the reaction of surrounding tissue upon implantation. Smaller pores can result in a strong inflammatory response that can reduce the growth of surrounding tissue. Polypropylene hernia mesh made with larger pores can reduce the inflammatory response but inhibit fibrous tissue growth.
Polypropylene mesh can also be manufactured using a knitting or weaving process which will influence the size of pores and flexibility. Knitted mesh is more porous and flexible, while the woven mesh is generally stronger – but less adept at promoting fibrous tissue growth.
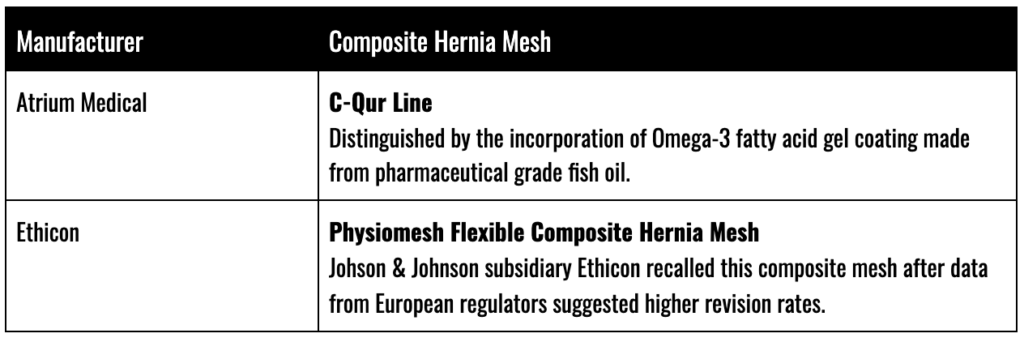
Permanent synthetic mesh materials like polypropylene are often combined with other absorbable materials to create “composite” meshes which, it is believed, will limit issues with inflammation, while promoting the strength and flexibility of the polypropylene. Typically, absorbable materials such as Dexon or Vicryl are used, which will completely degrade over time.
Examples of composite mesh products include:
Issues with Polypropylene in Hernia Repair
Most permanent meshes made with polypropylene were “fast-tracked” through the FDA’s 510(k) clearance process meaning that they went to market without rigorous testing or clinical trials. Many issues with synthetic permanent meshes were overlooked prior to implantation and many of them were not discovered until revealed in “post-market” surveillance required of manufacturers by the FDA. It is largely this type of ongoing review which has unmasked some of the more serious side effects and complications associated with polypropylene meshes.
Propylene Mesh Adhesion
Synthetic meshes made with polypropylene are associated with an increased risk of adhesion in patients. Adhesions are scar tissue that forms around the synthetic mesh and, in some instances, cause tissue in the abdomen to “stick” or bind together. This can result in small or large intestines clumping and pulling on each other causing intense chronic pain. Adhesion can also result in a serious abdominal infection known as peritonitis.
Adhesion can also cause either partial or complete bowel obstruction. Complete bowel obstruction can lead to infections such as sepsis and requires surgery to restore blood flow to the intestines.
Propylene Mesh Migration
Typically following laparoscopic procedures, polypropylene mesh can fail to properly adhere to surrounding tissue. This failure then leads to what is called “migration” as the mesh moves from its original insertion point and moves around the abdomen wreaking havoc on the abdominal cavity with infections and abscesses. In some cases, migration can only be remedied with additional surgery that can even be as dramatic as bowel resection.
Propylene Mesh Fistula
Fistulas are abnormal connections between abdominal organs and the intestines which can result when polypropylene mesh “erodes” into the gastrointestinal tract. Depending upon the location of a fistula, fluid can leak into body cavities and inhibit their normal performance. Fistulas can cause diarrhea or severe dehydration.
Propylene Mesh and Bowel Perforation
Bowel perforations are holes that develop in the large intestine which cause severe and even deadly abdominal infections when the contents spill out into the surrounding cavity. Surgeons will typically rush into surgery once a bowel perforation is detected and perform an ileostomy or colostomy to affect tissue healing.
Polypropylene Mesh Recalls and the FDA
Atrium C-Qur
The FDA warned Atrium, the manufacturer of the C-Qur mesh product line, of infection and sterility issues as far back as 2012. Most of the issues related to Atrium’s choice of the manufacturing production line and poor hygiene standards. After Atrium failed to remediate those issues, the FDA issued a Class II recall and sued the company over its poor quality control.
Ethicon/Johnson & Johnson Physiomesh Recall
Ethicon voluntarily recalled its Physiomesh products in 2016 on the heels of a spate of studies which suggested that the permanent synthetic meshes suffered from higher-than-normal rates of revision surgeries and complications.
Sources Cited (23)
1) “Past, Present and Future of Surgical Meshes: A Review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5618132/
2) “A History of Polypropylene Hernia Mesh and Its Many Problems” https://herniamesh.legalexaminer.com/technology/medical-devices-implants-technology/a-history-of-polypropylene-hernia-mesh-and-its-many-problems/
3) “Everything You Need To Know About Polypropylene (PP) Plastic” https://www.creativemechanisms.com/blog/all-about-polypropylene-pp-plastic
4) “Suture versus preperitoneal polypropylene mesh for elective umbilical hernia repairs” https://pubmed.ncbi.nlm.nih.gov/24980854/
5) “Current Issues and Challenges in Polypropylene Foaming: A Review” https://www.researchgate.net/publication/282672632_Current_Issues_and_Challenges_in_Polypropylene_Foaming_A_Review
6) “RECENT PROBLEMS AND FAILURES WITH PP MESH IMPLANTS HAVE LED TO THOUSANDS OF HERNIA AND SURGICAL MESH LAWSUITS” https://www.plasticexpert.com/plastic-technology/are-polypropylene-mesh-implants-safe/
7) “Surgical approach to abdominal wall defects: history and new trends” https://www.sciencedirect.com/science/article/pii/S1743919113600084
8) “Mesh infection in cases of polypropylene mesh hernioplasty” https://pubmed.ncbi.nlm.nih.gov/32096086/
9) “Complications of polypropylene mesh for the treatment of female pelvic floor disorders” https://pubmed.ncbi.nlm.nih.gov/21965260/
10) “Biologic versus Synthetic Mesh Reinforcement: What are the Pros and Cons?” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4477030/
11) “Polypropylene mesh implantation for hernia repair causes myeloid cell–driven persistent inflammation” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6413778/
12) “Mesh complications in female pelvic floor reconstructive surgery and their management: A systematic review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3424888/
13) “Urogynecologic Surgical Mesh Implants” https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants
14) “Polypropylene mesh implantation for hernia repair causes myeloid cell–driven persistent inflammation” https://insight.jci.org/articles/view/123862
15) “Recurrent urinary tract infection due to hernia mesh erosion into the bladder” https://www.researchgate.net/publication/7970758_Recurrent_urinary_tract_infection_due_to_hernia_mesh_erosion_into_the_bladder
16) “Surgical Mesh: Use and Complications in Women” https://my.clevelandclinic.org/health/articles/16298-surgical-mesh-use-and-complications-in-women
17) “Polypropylene mesh and systemic side effects in inguinal hernia repair: current evidence” https://pubmed.ncbi.nlm.nih.gov/30915679/
18) “Life after mesh: One patient’s harrowing experience with transvaginal mesh” https://www1.racgp.org.au/newsgp/clinical/life-after-mesh-one-patient-s-harrowing-experience
19) “Mesh Information for Patients – Frequently Asked Questions (FAQs)” https://centralcarolinasurgery.com/posts/news/mesh-information-for-patients-frequently-asked-questions-faqs/
20) “Mesh materials and hernia repair” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5571666/
21) “Late-onset Deep Mesh Infection: A Study of Eight Cases Detected from 2666 Consecutive Patients with Abdominal Wall Hernia Repairs” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4976579/
22) “The Many Types of Hernia Mesh Explained” https://michiganherniasurgery.com/posts/hernia-repair/the-many-types-of-hernia-mesh-explained/
23) “Hernia Surgical Mesh Implants: FDA Activities” https://www.fda.gov/medical-devices/hernia-surgical-mesh-implants/hernia-surgical-mesh-implants-fda-activities