Last Updated October 27, 2020
Attorneys across the United States are taking notice of claims that popular “wakefulness” medications, Provigil and Nuvigil may be associated with increased incidences of birth defects. Although at the moment, no multidistrict litigation (MDL) or class-action lawsuits have been filed relative to these claims of birth defects, attorneys have taken it upon themselves to investigate these issues further and are accepting cases.

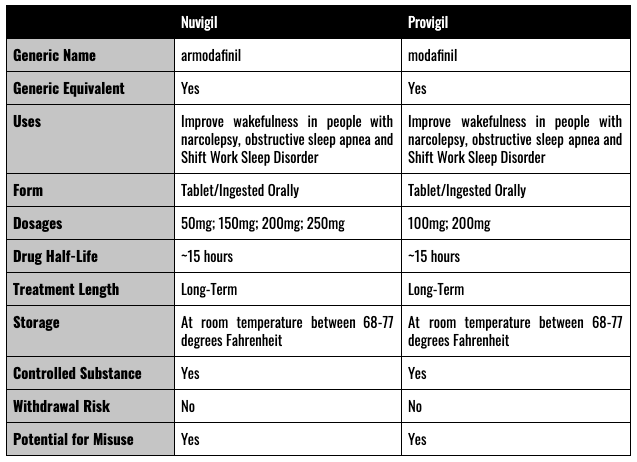
Provigil is the product name for the drug modafinil, an oral tablet that promotes wakefulness. Nuvigil is the product name for the drug armodafinil – also an oral tablet prescribed for patients having issues with daytime wakefulness. Provigil is manufactured by Teva Pharmaceuticals USA, Inc. and Nuvigil by Cephalon, Inc. (now a subsidiary of Teva). It is not known how either medication works to keep a person awake. However, they are thought to work by affecting certain substances in the brain that control the sleep and wake cycle through dopamine manipulation.
The FDA and Birth Defects
The U.S. Food and Drug Administration (FDA) classifies both Nuvigil and Provigil as “Category C” drugs. This means that the FDA has concerns about the use of both drugs during pregnancy, but will not state that a clear safety hazard exists on the basis of evidence. Nonetheless, both Nuvigil and Provigil have been associated with the following birth defects:
- Cleft Lip or Palate
- Hypospadias
- Congenital Heart Defects
- Microcephaly
In 2009, the FDA expressed enough concern about this association that it established a formal “pregnancy registry” for modafinil and armodafinil in order to gather additional information on the potential ramifications for pregnancy and fetal development.
Congenital Heart Defects
Congenital Heart Defects (also referred to as “CHD”) are present at birth in infants and can have a substantial impact on the function of how blood flows into a baby’s heart and through the rest of its body. Types of CHD include the following:
- Atrial Septal Defect
- Atrioventricular Septal Defect
- Coarctation of the Aorta
- Double-outlet Right Ventricle
- d-Transposition of the Great Arteries
- Ebstein Anomaly
- Hypoplastic Left Heart Syndrome
- Interrupted Aortic Arch
- Pulmonary Artesia
- Single Ventricle
- Tetralogy of Fallot
- Total Anomalous Pulmonary Venous Return
- Tricuspid Atresia
- Truncus Arteriosus
- Ventricular Septal Defect
Treatment options for CHD will depend upon the type and severity of the defect. Infants and children may require multiple surgeries to repair the heart or blood vessels. Furthermore, even though a heart defect can be repaired, treatment may not result in a cure. Children with CHD may develop other heart issues over time and require additional surgeries. With that in mind, however, many children born with CHD go on to lead independent lives with little or no difficulty.
Microcephaly
Microcephaly is a condition where a baby’s head is much smaller than is expected. This condition can occur because the baby’s brain has not developed properly during pregnancy or shortly after birth. In severe cases of microcephaly, a baby can be left with severe and life-threatening brain damage. Depending upon the severity, however, babies with microcephaly can also suffer from seizures; developmental delay; intellectual disability; problems with movement and balance; difficulty swallowing; hearing loss; and vision problems.
Microcephaly is a lifelong condition and there is no known cure or standard treatment. More severely affected babies will often require care and treatment focused on managing their health issues; as well as therapy to help maximize their physical and intellectual abilities.
Hypospadias
Hypospadias is a birth defect in boys impacting the position of the opening of the urethra. Babies born with hypospadias can form the opening to the urethra somewhere near the head of the penis (subcoronal); along the shaft of the penis (midshaft); or where the penis and the scrotum meet (penoscrotal). Additionally, hypospadias can result in curvature of the penis and abnormal spraying of urine. Hypospadias can be treated with surgery to correct the defect and is usually performed between the ages of 3-18 months old.
Cleft Lip and Cleft Palate
Children born with a cleft lip or cleft palate (sometimes in combination with each other) often have problems feeding and speaking clearly and may have regular ear infections. Both are birth defects that occur when a baby’s lip or mouth do not form properly during pregnancy. As a baby develops during pregnancy, facial tissue forms on each side of the head and join together to make the face. A cleft lip occurs when this tissue does not join completely before birth resulting in an opening in the upper lip. A cleft palate results when the tissue in the roof of the mouth similarly does not join together completely.
Depending upon the severity of the cleft and the existence of other birth defects, surgery can usually be an option for treatment within the first 12-18 months of life. With treatment, most children with clefts can expect to do well and live a happy life.
Anti-Trust Lawsuit
Although there are no large class-actions or MDLs involving birth defects yet, the manufacturers of Provigil and Nuvigil, Teva Pharmaceuticals, and Cephalon (along with generic manufacturers Mylan Laboratories and Ranbaxy Pharmaceuticals) recently paid over $134 million to settle an anti-trust lawsuit brought by the State of California. The lawsuit accused the companies of colluding to keep a generic version of Provigil from coming to the market sooner – a move which would have benefited consumers.
Sources Cited (16)
1. “The Nuvigil and Provigil Pregnancy Registry” https://clinicaltrials.gov/ct2/show/NCT01792583
2. “DailyMed: Nuvigil – Armodafinil” https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d878aed0-ddbf-8fa1-abf7-d3e480260845
3. “State of California v. Teva Pharmaceuticals, et al.” https://oag.ca.gov/sites/all/files/agweb/pdfs/antitrust/provigilsettlement/complaint.pdf
4. “Taking Provigil While Pregnant: What You Need to Know” https://www.therecoveryvillage.com/provigil-addiction/provigil-while-pregnant/
5. “Facts about Cleft Lip and Cleft Palate” https://www.cdc.gov/ncbddd/birthdefects/cleftlip.html
6. “Facts about Hypospadias” https://www.cdc.gov/ncbddd/birthdefects/hypospadias.html
7. “Facts about Microcephaly” https://www.cdc.gov/ncbddd/birthdefects/microcephaly.html
8. “The Safety of Stimulant Medication Use in Cardiovascular and Arrhythmia Patients” https://www.acc.org/latest-in-cardiology/articles/2015/04/28/10/06/the-safety-of-stimulant-medication-use-in-cardiovascular-and-arrhythmia-patients
9. “First-Trimester Pregnancy Exposure to Modafinil and Risk of Congenital Malformations” https://jamanetwork.com/journals/jama/fullarticle/2759460
10. “What are Congenital Heart Defects?” https://www.cdc.gov/ncbddd/heartdefects/facts.html
11. “Modafinil: Potential Risk of Congenital Malformations When Administered During Pregnancy” http://www.hpra.ie/docs/default-source/default-document-library/important-safety-information—modafinil99170c2697826eee9b55ff00008c97d0.pdf
12. “Practical Use and Risk of Modafinil, a Novel Waking Drug” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3286657/
13. “Health Canada Warns that Modafinil May Cause Fetal Harm” https://aasm.org/health-canada-warns-modafinil-fetal-harm/
14. “FDA Pregnancy Categories” https://chemm.nlm.nih.gov/pregnancycategories.htm
15. “Armodafinil While Pregnant: What You Need to Know” https://www.therecoveryvillage.com/armodafinil-addiction/armodafinil-while-pregnant/#gref
16. “Mechanisms of Modafinil: A Review of Current Research” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2654794/