According to the results of a new longitudinal study, using e-cigarettes and other tobacco products to prevent smoking relapse is not effective. Nearly 13,000 smokers in the United States participated in this research. It is the first study to report on whether cigarette smokers can switch to e-cigarettes and successfully quit using cigarettes.
Use of E-Cigarettes to Prevent Smoking Relapse
“Quitting is the most important thing a smoker can do to improve their health,” explained study author John Pierce. “But the evidence indicates that switching to e-cigarettes made it less likely, not more likely, to stay off of cigarettes.”
Specifically, the research found that 50 percent of former smokers who quit “cold turkey” were still non-smokers as of the second annual follow-up. Only 41.5 percent of former smokers who used an alternative form of tobacco in their quitting efforts found success. “Those who switched to e-cigarettes (or indeed another form of tobacco) were 8.5 percent more likely to relapse to cigarettes,” said Pierce, a professor of family medicine and public health at the UC San Diego Institute for Public Health.
The study did not consider the use of nicotine replacement therapy (NRT), which is specifically designed to help people stop smoking. Those therapies include patches, gum, and lozenges that contain limited amounts of nicotine.
Harmful History of Vaping
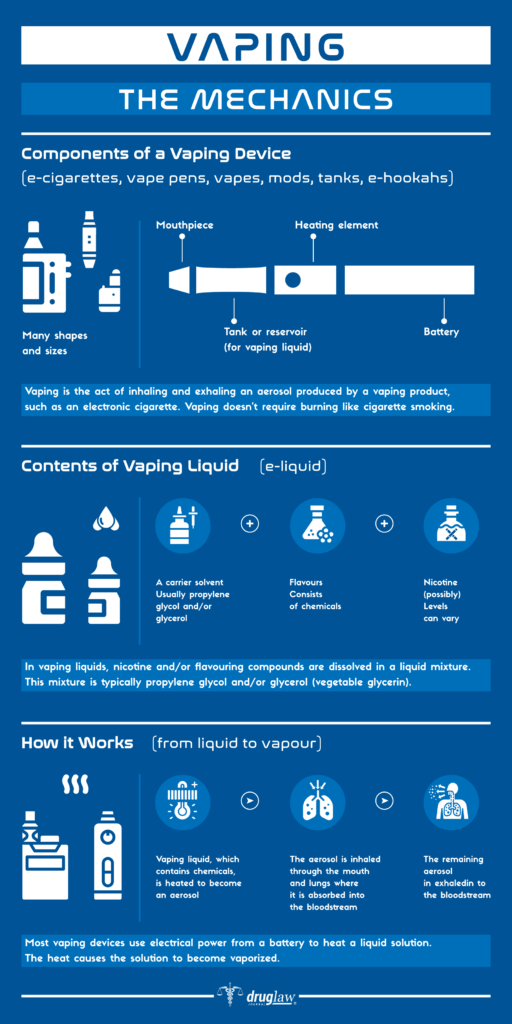
E-cigarettes are often used a nicotine alternative for smokers trying to quit. They work by using an often-flavored liquid called e-juice, which eventually vaporizes. Vaping eliminates the over 7,000 chemicals, including the toxic ones, that are found in a burning cigarette.
Vaping became popular for tobacco cessation after a study out of the United Kingdom found that e-cigarettes helped as many as 70,000 smokers in England quit smoking in 2017. However, their use is now controversial due to potential long-term health effects from chemicals in the vape juice or cartridge.
Additionally, multiple studies determined that teen vaping often leads to cigarette smoking. American youth also dealt with a serious outbreak of a vaping-related lung disease, now known as EVALI (e-cigarette or vaping use-associated lung injury), in 2019. According to the Centers for Disease Control and Prevention, as of February 2020, the illness had killed at least 68 people and sickened over 28,000 e-cigarette users.
For more information about the investigation into e-cigarettes, contact us today.
Additional Reading:
Big Drop in Youth Vaping Thanks to COVID School Closures
FDA Authorizes Vuse Solo E-Cigarette, Shows Benefit for Smokers
FDA Requests More Time to Determine Ruling About E-Cigarettes
FDA Removes 55,000 Flavored Vaping Products From Market