Last Updated November 2, 2020
Valsartan is a popular anti-hypertensive drug that has been in widespread use since the 1990s. It can be used alone or in conjunction with other medications to combat high blood pressure/hypertension or to aid in recuperation following a heart attack episode. In recent years, as generic manufacturers moved into the market for Valsartan, they took the production of this drug into factories located in India and the People’s Republic of China. The decision to move the manufacturing of generic varieties of Valsartan offshore was a fateful one as it was later discovered that some batches made in these factories were contaminated with potent carcinogens which later found their way into the U.S. drug supply.

Beginning in 2018, the U.S. Food and Drug Administration (FDA) began wide-ranging recalls of generic Valsartan. Now, individuals suffering from various forms of cancer are suing these generic manufacturers and their lawsuits have been consolidated into a large multidistrict litigation (MDL-2875) taking place in federal court in New Jersey.
Contamination of Generic Valsartan Supplies
Valsartan is manufactured in generic formats by firms around the world. Most of the “Active Pharmaceutical Ingredient” (API) for generic Valsartan is itself subcontracted out to a handful of factories in the People’s Republic of China and India. FDA testing of certain generic brands of Valsartan revealed that some batches were contaminated with N-Nitrosodimethylamine (NDMA), an extremely potent carcinogen. In 2018, the FDA announced recalls of contaminated batches of Valsartan as well as other similar medications such as Losartan and Irbesartan. The complete list of recalled batches and manufacturers can be found here.
It is believed that improper manufacturing processes at plants in Zhejiang, China, and Telangana, India, are to blame for NDMA contamination. These factories were outsourced by generic drug manufacturers to maximize the profitability of their brands and operated with little or no oversight or monitoring for safety and quality. Consequently, contaminated medications made their way through the supply chain and into batches circulated through the United States, Canada, and Europe.
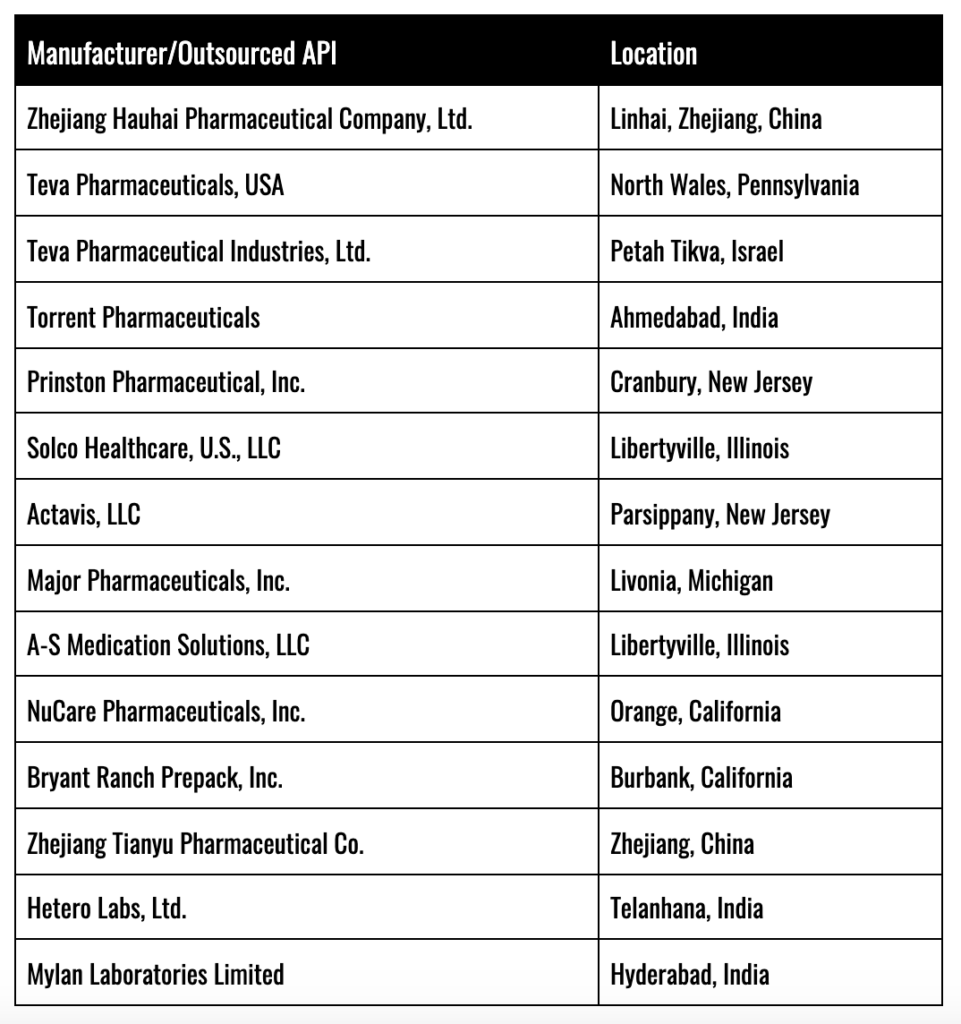
Here is a list of some of the larger generic Valsartan manufacturers:
What Are the Risks from NDMA Contamination?
NDMA is a Group 2A carcinogen meaning that it is likely to cause cancer in humans because research has shown it to cause cancer in animals. Animals exposed to NDMA for periods over several weeks tended to show some development of liver and lung cancers as well as other types of cancer.
A prospective study published in a 2011 issue of the American Journal of Clinical Nutrition found that NDMA is associated with a higher incidence of gastrointestinal cancer as well as rectal cancer in humans. Other studies have linked NDMA to higher incidences of esophageal, oral cavity, and pharynx cancers.

Valsartan Lawsuits
The first lawsuits naming manufacturers for their role in placing contaminated Valsartan into the drug supply began cropping up in state courts in 2018. Many of these lawsuits were removed to federal courts and in 2019 consolidated into single multidistrict litigation (MDL-2875). As of August 2020, there were 339 plaintiffs involved in this MDL.
The majority of the complaints filed by injured parties in the MDL assert that they were prescribed certain generic forms of Valsartan which were contaminated with NDMA and that in turn, they believe that the contamination is behind their cancer diagnosis. In particular, some claimants allege that contaminated Valsartan is behind a range of gastrointestinal and gastroesophageal cancers as well as liver and pancreatic cancers. Finally, claimants allege that the manufacturers were, at a minimum, negligent in their manufacturing processes and safety/quality control standards.
It is important to note that not all batches of generic Valsartan were contaminated. Lawsuits are focused entirely on generic batches that were subject to FDA recall. The domestic brands of Valsartan, such as those marketed by Novartis, under the brand name Diovan, are not part of the FDA recall.
Additionally, some potential claims alleging illness from contaminated generic Valsartan may be thwarted by the applicable statute of limitations in each state. Accordingly, if you believe you have taken contaminated generic Valsartan and have suffered an injury, you should consult with an attorney as soon as possible. An attorney can advise you about the statute of limitations and other matters that could affect your ability to file.

Sources Cited (16):
1) “In re: Valsartan NDMA Contamination Litigation” https://ecf.jpml.uscourts.gov/doc1/8501984141
2) “Leanne Gentry et al. v. Solco Healthcare U.S., LLC et al.” https://ecf.mowd.uscourts.gov/doc1/10916957329
3) Use of N-nitrosodimethylamine (NDMA) contaminated valsartan products and risk of cancer: Danish nationwide cohort study” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6134800/#:~:text=Valsartan%20is%20an%20angiotensin%20II,treat%20hypertension%20and%20heart%20failure.&text=In%20July%202018%2C%20some%20valsartan,N%2Dnitrosodimethylamine%20(NDMA).
4) FDA Updates and Press Announcements on Angiotensin II Receptor Blocker (ARB) Recalls (Valsartan, Losartan, and Irbesartan)” https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan
5) “Plaintiffs: Valsartan Manufacturers Should Have Known About NDMA Contamination” https://newyork.legalexaminer.com/health/plaintiffs-valsartan-manufacturers-should-have-known-about-ndma-contamination/
6) “Search List of Recalled Angiotensin II Receptor Blockers (ARBs) including Valsartan, Losartan and Irbesartan” https://www.fda.gov/drugs/drug-safety-and-availability/search-list-recalled-angiotensin-ii-receptor-blockers-arbs-including-valsartan-losartan-and
7) “FDA Expands Valsartan Products Recall” https://www.pharmacytimes.com/resource-centers/cardiovascular-health/fda-expands-valsartan-products-recall
8) “Valsartan, Losartan & Other BP Med Recalls 2018-19” https://www.webmd.com/hypertension-high-blood-pressure/valsartan-losatran-bp-med-recalls-2018-19
9) “NDMA, a contaminant found in multiple drugs, has industry seeking sources and solutions” https://cen.acs.org/pharmaceuticals/pharmaceutical-chemicals/NDMA-contaminant-found-multiple-drugs/98/i15
10) “What We Know about the Possible Carcinogen Found in Zantac” https://www.scientificamerican.com/article/what-we-know-about-the-possible-carcinogen-found-in-zantac/
11) “Does NDMA Cause Cancer?” https://www.verywellhealth.com/ndma-cancer-risk-5083965
12) “N-Nitrosodimethylamine” https://pubchem.ncbi.nlm.nih.gov/compound/N-Nitrosodimethylamine
13) “Use of N-nitrosodimethylamine (NDMA) contaminated valsartan products and risk of cancer: Danish nationwide cohort study” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6134800/
14) “A survey of feeding N-nitrosodimethylamine (NDMA) to domestic animals over an 18 year period” https://pubmed.ncbi.nlm.nih.gov/7228298/
15) “Dietary intakes of nitrate, nitrite and NDMA in the Finnish Mobile Clinic Health Examination Survey” https://pubmed.ncbi.nlm.nih.gov/8799716/
16) “Inflammatory bowel disease stimulates formation of carcinogenic N-nitroso compounds” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1774505/


