Last Updated December 18, 2020
Zantac is the brand name for ranitidine, a very popular medication used for the treatment of issues associated with heartburn, ulcers, and Gastro-Esophageal Reflux Disease (GERD). Ranitidine is part of a larger family of drugs known as histamine H2-receptor antagonists or “H2-blockers” for their method of action in blocking the action of histamine cells in the stomach lining.
Approved in 1983 for prescription use by the U.S. Food and Drug Administration (FDA), Zantac was wildly successful and by 1986 netted over $1 billion in sales for pharmaceutical giant, GlaxoSmithKline – making it one of biggest runaway hits in history. Zantac eventually went over-the-counter (OTC) in 1996 and following its move “off-patent” became an equally successful generic drug. In 2018, Zantac (ranitidine) ranked as one of the top-10 antacid tablets in the United States.
In 2019, things began to unravel for Zantac and the story of its meteoric rise to pharmaceutical sensation. A small, upstart testing laboratory in Connecticut revealed the presence of N-Nitrosodimethylamine, a potent carcinogen also known as “NDMA” in batches it sampled. It is now believed that the NDMA in Zantac may be the result of natural breakdown processes in ranitidine itself which potentially expands the range of exposure to nearly every person who has ever taken Zantac. In April 2020, the FDA announced a total recall of all Zantac (ranitidine) products from the market.
How does Zantac Function?
Histamine is a naturally occurring chemical present throughout the body which interacts with the cells in the stomach lining to promote the production of stomach acid. Drugs like Zantac work to block the histamine receptors traveling through the stomach, thereby limiting the signal to produce stomach acid.
How Long Does It Take Zantac to Work?
Zantac is absorbed into the bloodstream, unlike more traditional calcium carbonate antacids. However, in contrast to proton pump inhibitors (PPIs) which can take several days or weeks to activate, it will begin to work and provide relief of acid indigestion and heartburn within 1-2 hours.
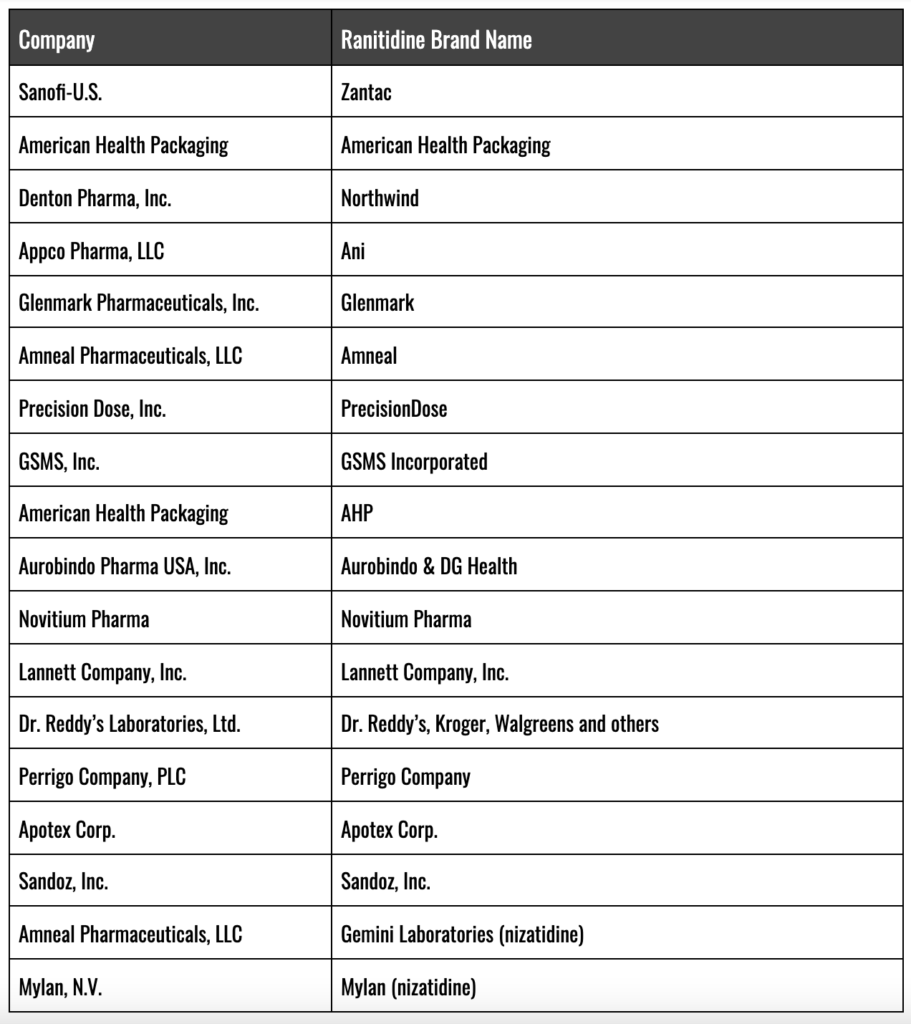
How Many Brands of Zantac (ranitidine) Were on the Market at the Time of Recall?
The following is a list of branded Zantac and generic ranitidine brands at the time of recall:
What Are the Common Side Effects of Zantac?
Common Zantac side effects include, but are not limited to:
- Insomnia
- Impotence
- Difficulty achieving orgasm
- Muscle pain
- Stomach pain
- Nausea
- Vomiting
- Swollen or tender breasts (in men)
- Constipation
- Diarrhea
- Fatigue
- Headache
- Drowsiness
- Dizziness
What is the Risk of Cancer from NDMA?
The International Agency for Research on Cancer (IARC) classifies NDMA as a Group 2A carcinogen which means they believe it is “probably carcinogenic to humans”. Specifically, it is believed that NDMA causes mutations in human DNA which can lead to harmful cancers. In humans intentionally poisoned with NDMA, severe liver damage and internal bleeding have resulted. Animal studies have revealed NDMA to be toxic even after short-term exposure.
What Cancers Are Associated with NDMA?
Research into NDMA and human exposure have yet to draw a definitive link between NDMA, Zantac, and cancer. The causes of cancers in humans are numerous and can range from factors involving behaviors such as smoking and drinking to a genetic predisposition to environmental factors and obesity. Accordingly, it is difficult to put a precise finger on what cancers could develop from NDMA contamination in Zantac. Nonetheless, researchers have theorized that long-term exposure through Zantac could be associated with the following types of cancer:
- Stomach
- Bladder
- Colorectal
- Liver
- Esophageal
- Small Intestinal
Although less prevalent in research, NDMA has also been linked to these types of cancers:
- Prostate
- Ovarian
- Pancreatic
- Lung
- Uterine
- Brain
- Breast
- Thyroid
- Testicular
- Leukemia
- Non-Hodgkin’s Lymphoma
- Multiple Myeloma
Does Zantac Interact with Other Medications?
Research has documented observations of Zantac interacting with common blood-thinners such as Warfarin. This interaction resulted in fluctuating levels of a blood test measuring prothrombin timing. Additionally, studies of Zantac suggest the possibility that it could interact with acetaminophen, ibuprofen, and metformin.
Is It True that Zantac Manufacturers Are Involved in Lawsuits?
Yes. In 2019, several individuals suffering from cancer who believe that their illness stems from taking Zantac contaminated with NDMA filed lawsuits against manufacturers such as:
- Boehringer Ingelheim Pharmaceuticals, Inc.
- Sanofi Aventis, S.A./Sanofi-U.S. Services, Inc.
- Chattem, Inc.
- Pfizer, Inc.
- GlaxoSmithKline, Inc.
Cases have now been consolidated into single multidistrict litigation (MDL-2924) taking place in West Palm Beach, Florida. New cases are being added at this time.
Sources Cited (15):
1) “Popular heartburn drug ranitidine recalled: What you need to know and do” https://www.health.harvard.edu/blog/popular-heartburn-drug-ranitidine-recalled-what-you-need-to-know-and-do-2019092817911
2) “Technical Fact Sheet – N-Nitroso-dimethylamine (NDMA)” https://www.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_contaminant_ndma_january2014_final.pdf
3) “The Zantac problem: What’s NDMA?” https://abcnews.go.com/Health/zantac-problem-whats-ndma/story?id=65799147
4) “FDA Requests Removal of All Ranitidine Products (Zantac) from the Market” https://www.fda.gov/news-events/press-announcements/fda-requests-removal-all-ranitidine-products-zantac-market
5) “FDA Updates and Press Announcements on NDMA in Zantac (ranitidine)” https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-ndma-zantac-ranitidine
6) “Recalls, Market Withdrawals, & Safety Alerts” https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts
7) “NDMA, a contaminant found in multiple drugs, has industry seeking sources and solutions” https://cen.acs.org/pharmaceuticals/pharmaceutical-chemicals/NDMA-contaminant-found-multiple-drugs/98/i15
8) “Ranitidine Cancer Risk“ https://www.medpagetoday.com/meetingcoverage/ddw/86314
9) “Questions and Answers: NDMA impurities in ranitidine (commonly known as Zantac)” https://www.fda.gov/drugs/drug-safety-and-availability/questions-and-answers-ndma-impurities-ranitidine-commonly-known-zantac#:~:text=FDA%20has%20found%20N%2Dnitrosodimethylamine,ranitidine%20they%20may%20currently%20have.
10) “Hansen v. Boehringer Ingelheim Pharmaceuticals, Inc., et al” https://ecf.caed.uscourts.gov/doc1/033111300340
11) “Balisteri v. Boehringer Ingelheim Pharmaceuticals, Inc., et al” https://ecf.cand.uscourts.gov/doc1/035118602775
12) “Our Mission” https://www.valisure.com/about-us/
13) “Formation Mechanism of NDMA from Ranitidine, Trimethylamine, and Other Tertiary Amines during Chloramination: A Computational Study” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4123930/
14) “Statement alerting patients and health care professionals of NDMA found in samples of ranitidine” https://www.fda.gov/news-events/press-announcements/statement-alerting-patients-and-health-care-professionals-ndma-found-samples-ranitidine
15) “Clinical Study to Investigate the Urinary Excretion of N-nitrosodimethylamine (NDMA) After Ranitidine Administration” https://clinicaltrials.gov/ct2/show/NCT04397445