The Inferior Vena Cava is the single-largest vein in the human body and it bears the responsibility for moving blood from your lower body up to the cardiopulmonary system in the chest cavity. It is part of a larger network of veins in the vascular system that return oxygen-poor blood and waste products from the rest of the body back to the heart. Over time, these veins can develop areas of thickened blood that clump together to form what are called “clots”. Clots that form below the heart usually form inside the deep veins of the thigh or lower leg and are commonly known as “Deep Vein Thrombosis” or DVT. DVT can cause swelling, pain, and tenderness in the leg. However, a DVT can also break loose and make its way to the lung and cause a potentially life-threatening condition known as Pulmonary Embolism or PE. Accordingly, Inferior Vena Cava (IVC) filters are small mechanical devices placed into the IVC in order to prevent DVT and other types of blood clots from using this vascular highway to traverse the body and into the lungs.
Between 1999 and 2008, there was a dramatic increase in the number of filters implanted in the United States – growing from 49,000 to 140,000 over the period. On the one hand, the growth in these numbers partially reflects the technical and engineering advances made by device manufacturers. On the other hand, it also reflects a rapid push into the marketplace by a number of firms eager to capitalize on this growing patient segment. Along the way, the American medical establishment has been subjected to criticism for resorting to IVC filters rather than less-invasive treatments involving blood-thinners and other protocols.
What Is an IVC Filter and How Does It Work?
An IVC filter is a small and spindly looking device which is surgically placed inside of the IVC to sift and clear blood components passing through it. Blood flows through the IVC and the filter on its way back to the heart and lungs. The filter acts to intercept and catch clots before they reach areas where they can cause serious damage.
The procedure for implanting an IVC filter typically involves a smaller incision made by an implanting physician in either the groin or neck. The doctor will then insert a flexible tube or “catheter” with a collapsed filter into a vein and route into the IVC. Once it reaches the IVC, the filter is then triggered to expand and attach itself to the walls of the vein. In some cases, the filter becomes a permanent fixture in the IVC, although others are implanted with the understanding they will be removed after some period of time.
Who Might Need an IVC Filter?
IVC filters tend to be recommended or “indicated” for patients who suffer from a DVT diagnosis and who cannot take blood-thinning medication due to an elevated risk of bleeding or if they are queued for a surgical procedure that will require them to suspend taking blood thinners temporarily.
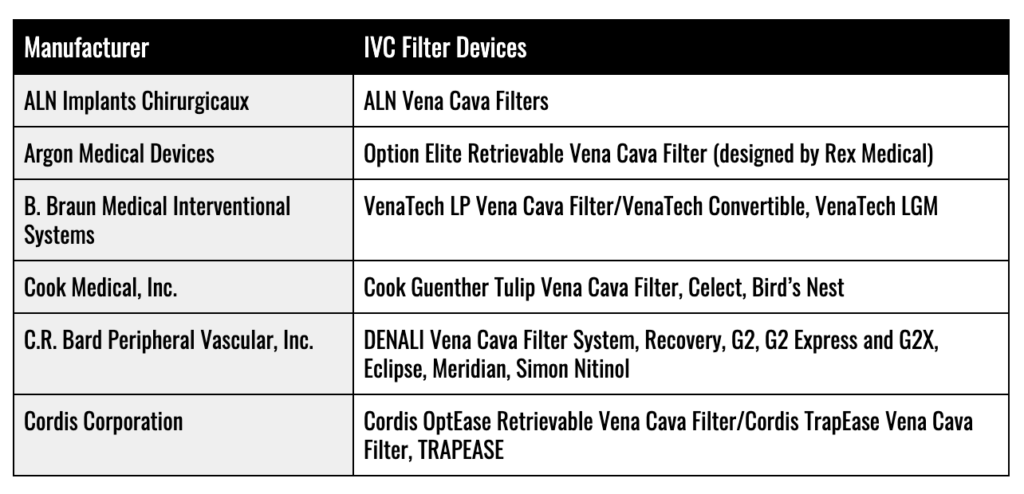
IVC Filter Manufacturers
Types of IVC Filter
There are two general types of IVC filters on the market in the United States: permanent and optional (also known as “retrievable”). Permanent filters have been utilized in patients since the 1970s and are placed in patients with long-term needs to filter against PE or other issues with blood clotting. Retrievable filters are newer and as the name suggests, are designed to be retrieved from the patient (or sometimes left in place) after a temporary risk of PE or clotting has passed or been resolved.
In theory, retrievable filters have the benefit of fewer long-term complications associated with filters left in the body on a permanent basis. With that in mind, retrievable filters have dramatically altered the thinking about using filters among medical professionals. The availability of retrievable filters has led to an increase in filter placement and the number of indications where they are now deemed suitable. However, the thinking and doctrine behind the removal of retrievable filters have not kept up with their increased placement as many patients find that their retrievable filters are left in their bodies anyway.
Issues Associated with IVC Filters
As noted above, IVC filters have enjoyed widespread adoption and use – in particular following the introduction of optional or retrievable filters in the 1990s. However, with the increased use of the filter, has come a growing recognition that there are a number of safety complications associated with these devices. Specifically, experts are concerned that retrievable filters are now potentially overused and that up to two-thirds of retrievable devices implanted in patients are never removed.
Filter migration is a serious problem associated with filter devices left in the body for too long. Migration occurs when an IVC filter shifts more than one centimeter in either direction. There are three main types of IVC filter migration:
- Mechanical: Typically involves the failure of the filter device itself.
- Iatrogenic: Filters are measured and approved to fit specific IVC measurements. Iatrogenic migration occurs where the device doesn’t fit the placement properly due to guide wire entanglement.
- Physiological: Actions such as bending, coughing, or straining have the potential to dislodge an IVC filter.
Filter Warnings, Voluntary Withdrawals, and Lawsuits
The dramatic jump in the number of implanted IVC filters in recent years has not escaped the attention of the U.S. Food and Drug Administration (FDA). In 2010, the FDA issued its first warning about IVC filters on the heels of a spike in “adverse event reports”. These reports detailed over 921 injuries from filters, including:
- 328 reports of filter migration
- 146 reports of filter migration into the heart and/or lungs (embolization)
- 70 reports of filter perforation of the vena cava
- 56 reports of filter fracturing
In May 2014, the FDA issued a “safety communication” concerning retrievable IVC filters. In addition to the agency’s observations from the 2010 FDA communique, the agency mandated that filter manufacturers participate in the collection of additional clinical data on filters currently marketed in the United States. Manufacturers were then given the option of either participating in the agency’s 522 Postmarket Surveillance Studies program or an independent national clinical study known as Predicting the Safety and Effectiveness of Inferior Vena Cava Filters (PRESERVE). The FDA will use data from both sources to evaluate growing patterns of filter use and to make additional recommendations concerning patient care.
The FDA has yet to issue a mandatory recall for any IVC filter product. However, between 2005 and 2019 at least eight (8) types of IVC filters have been voluntarily withdrawn from the market by their manufacturers over safety concerns. Filter manufacturer C.R. Bard was recently party to a now-settled case in multidistrict litigation over its filter products (MDL-2641). Cook Medical is currently a party to ongoing multidistrict litigation in federal court in Indiana (MDL-2570) over its IVC filter products.

Sources Cited (20):
1) “Inferior Vena Cava Filter Placement” https://emedicine.medscape.com/article/1377859-overview
2) “Decision analysis of retrievable inferior vena cava filters in patients without pulmonary embolism” https://www.fda.gov/media/88760/download
3) “Evidence-Based Evaluation of Inferior Vena Cava Filter Complications Based on Filter Type” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862854/
4) “Inferior Vena Cava (IVC) Filter Placement” https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/inferior-vena-cava-ivc-filter-placement
5) “Long-Term Clinical Outcomes of Complicated Retrievable Inferior Vena Cava Filter for Deep Venous Thrombosis Patients: Safety and Effectiveness” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6330022/
6) “Inferior Vena Cava Filter Placement and Removal” https://www.radiologyinfo.org/en/info.cfm?pg=venacavafilter
7) “Filter tilting and retrievability of the Celect and Denali inferior vena cava filters using propensity score-matching analysis” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6134324/
8) “About Your Inferior Vena Cava (IVC) Filter Placement” https://www.mskcc.org/cancer-care/patient-education/ivc-filter-placement
9) “What is the evidence behind the IVC filter?” https://emcrit.org/pulmcrit/what-is-the-evidence-behind-the-ivc-filter/#:~:text=37%20patients%20with%20recurrent%20venous,rate%20of%20IVC%20filter%20thrombosis.&text=Therefore%2C%20they%20concluded%20that%20this,two%20problems%20with%20this%20argument.
10) “Vena Cava Filters” https://my.clevelandclinic.org/health/treatments/17609-vena-cava-filters
11) “Vena cava filters: Tiny cages that trap blood clots” https://www.health.harvard.edu/heart-health/vena-cava-filters-tiny-cages-that-trap-blood-clots
12) “Inferior Vena Cava Filters: Guidelines, Best Practice, and Expanding Indications” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862857/#:~:text=There%20are%20two%20general%20types,and%20absolute%20contraindications%20to%20anticoagulation.
13) “Inferior Vena Cava Filter” https://www.ncbi.nlm.nih.gov/books/NBK549900/
14) “Permanent versus Retrievable Inferior Vena Cava Filters: Rethinking the “One-Filter-for-All” Approach to Mechanical Thromboembolic Prophylaxis” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862850/
15) “FDA Issues Statement on Treatment and Follow-Up Regarding IVC Filter Complications” https://evtoday.com/news/fda-issues-statement-on-treatment-and-follow-up-regarding-ivc-filter-complications
16) “Predicting the Safety and Effectiveness of Inferior Vena Cava Filters” http://www.preservetrial.com/
17) “Vena cava filters” https://www.medicalexpo.com/medical-manufacturer/vena-cava-filter-44592.html
18) “Complications of Inferior Vena Caval Filters” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3036364/
19) “IVC Filter Migration: How Does It Happen?” https://medium.com/@Drug_Justice/ivc-filter-migration-how-does-it-happen-1e453d77a0af
20) “FDA Updates Safety Communication on IVC Filter Retrieval” https://evtoday.com/news/fda-updates-safety-communication-on-ivc-filter-retrieval




