Last Updated December 2, 2020
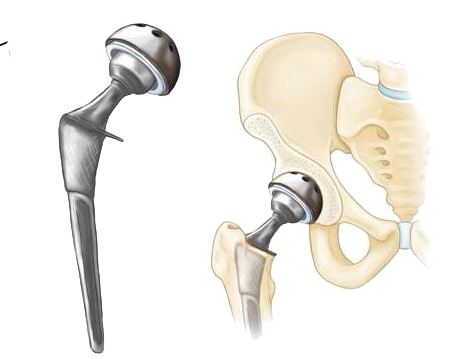
Generally, hip placement surgery – also known as Total Hip Arthroplasty (THA) enjoys a favorable rate of success in patients. However, even under the best of circumstances, THA can still fail for any number of reasons. If a hip replacement procedure is unsuccessful or does not otherwise meet expectations, a surgeon may recommend a follow-up procedure to either remove or replace some or all of the implanted device. This type of surgery is known as a “revision”
Reasons for Hip Revision Surgery
Patients who undergo THA can usually expect that their prosthesis will last at least 15-20 years – and in some cases, remain within the patient for the rest of their life. However, in approximately 18% of all hip replacement scenarios, doctors will recommend revision surgery. The most typical factors mitigating in favor of revision are:
Recurrent or Repetitive Dislocation of the Hip Joint
Traumatic hip dislocation can occur where the ball of the hip joint is pushed out of the socket. A dislocated hip can be debilitating if not addressed to put the ball and socket back into proper alignment and ensure proper complementation by surrounding muscle and ligament tissue.
Fortunately, dislocation is a relatively infrequent phenomenon among younger patients who follow proper care guidelines given by their physician. However, among elderly patients or those who have THA following fracture, rates of dislocation rise precipitously. Furthermore, patients who have already suffered dislocation are more likely to suffer additional dislocations without corrective surgery as surrounding tissue becomes increasingly disrupted.
Infection
Infection resulting from any surgical setting, including hip replacement, is a very real problem and a possibility all patients must face. Exposure to infectious bacterial agents can occur even in surgical theaters observing even the most stringent sterilization protocols. Additionally, patients who suffer from conditions such as diabetes mellitus, obesity, peripheral vascular disease, or are immunocompromised, may be at even greater than usual risk for infection.
The risk for infection is greatest within the first six weeks following surgery. If infection sets into the prosthesis, the surgeon will usually recommend a battery of tests to identify the type of infectious agent at work, possibly including an aspiration of joint fluid from the hip joint itself.
Once the surgical team identifies the type of infection, they will consider a range of treatment options. Chief among these is the use of targeted antibiotics and revision surgery to eradicate the infection. Typically revision procedures to address infection include:
- Surgical cleansing with intravenous antibiotics.
- A complete exchange of the hip replacement device:
- Two-Stage: The surgeon will remove the device and infection then clean the bone. A temporary cement spacer will be implanted while the patient undergoes a six-week course of intravenous antibiotics. Following the antibiotic treatment, a new prosthetic device will be implanted.
- Single Stage: This procedure entails the removal of the device, total cleansing of the infected joint, and replacement with a new device all at once. Like the two-stage procedure, patients will require six-to-eight weeks of intravenous antibiotics afterward.
Wear and Tear/Loosening
The metal, ceramic and plastic components of a hip joint replacement will rub against each other as part of the normal function of the prosthesis. Over time, however, joint movement can slowly wear down parts of the implant increasing the risk of failure or revision.
Typical factors contributing to excessive wear and/or loosening include:
- Excessive vigorous physical activity by younger/more active patients
- Obesity
- The type of device (i.e. “metal on metal” vs. “metal on polyethylene” vs “metal on ceramic”)
In some cases, wear and tear from the device will introduce synthetic particles into surrounding tissue as well as the patient’s bloodstream. These particles can then trigger an immune response that fosters progressively worse conditions such as osteolysis (destruction of surrounding bone tissue).
In Metal-on-Metal (MoM) hip implants, the metal ball and metal cup slide against each other during walking or running. This friction between the ball and socket can cause the release of metal particles which will wear off of the device and embed in surrounding tissue as well as metal ions of cobalt and chromium entering the bloodstream. Over time, this leaching of metal from the joint into surrounding tissue causes “Adverse Local Tissue Reaction” (ALTR) and/or “Adverse Reaction to Metal Debris” (ARMD). The bottom line for patients implanted with MoM joints, however, is that they suffer damage and pain, device failure, loosening, and the need for revision surgery. Furthermore, patients implanted with MoM hip joints can also suffer from a condition known as “metallosis” – a potentially fatal complication arising from metallic erosion in the joint which induces pain around the joint, pseudotumors, and a noticeable rash.
What to Expect with Hip Revision Surgery
Pre-Operative Protocols
In the weeks and days prior to revision surgery, doctors will order a variety of tests such as X-rays, CT and bone scans to identify the issues which they seek to address during the procedure. Additionally, they may ask for a routine aspiration of the joint to rule out infection risks prior to surgery. Patients should make arrangements for post-surgical assistance around the home and consult with their personal physician concerning medications or other relevant physical conditions. It is recommended that patients stop smoking as long as possible before surgery.
The Revision Procedure
A hip revision can be a longer and more complex procedure than the original THA surgery. A simple revision can just be a swap of liner components. A more complex revision can be as involved as a total replacement of all components. However, effective planning and coordination between the surgical team and your personal physician can help ward off more serious complications during and after the revision procedure.
Post-Operative Matters
Following revision surgery, patients may be allowed to sit up out of bed or even walk the very next day upon the advice of their surgeon. Some pain, but not excessive pain, is to be expected. Patients are typically discharged to their home or to a rehabilitation hospital within 5-7 days post-revision. Afterward, they will usually transition into a physical therapy regimen accompanied by the use of crutches and canes for walking assistance for as many as six weeks.
Complications and Risks from Revision Surgery
Revision surgery, like other major procedures, is not without inherent risk. Possible medical complications associated with any surgery include, but are not limited to:
- Allergic Reactions to Certain Medications
- Blood Loss
- Heart Attacks/Strokes/Kidney Failure/Pneumonia
- Nerve Damage
Risks more commonly associated with THA and revision surgery also include:
- Deep Vein Thrombosis (DVT) is a widely recognized risk factor following hip revision surgery. DVT is a blood clot in the leg that can break loose and become a Pulmonary Embolism (PE) that travels through the body and into the lungs where it can cause illness and death. DVT can occur in any vein in the body, however, they tend to manifest the most often in the lower extremities of the body.
- Infection following revision surgery is a possibility and a very serious problem. Accidental exposure to bacteria such as staphylococcus aureus can occur even in the midst of the most stringent sterilization protocols. Common postoperative infection symptoms include swelling, stiffness, pain, redness, fevers, night sweats, and chills.
Metal-on-Metal Hip Implants and Higher Rates of Revision Surgery
Starting in the early 2000s, several manufacturers aggressively marketed hip-joints through the 510(k) process featuring a “Metal-on-Metal” (MoM) coupling feature between the ball and socket of the joint. In MoM joints, the metal ball and metal cup slide against each other during walking or running. This friction between the ball and socket can cause the release of metal particles which will wear off of the device and embed in surrounding tissue as well as metal ions of cobalt and chromium entering the bloodstream.
The bottom line for patients implanted with MoM joints is that they suffer damage and pain, device failure, loosening, and the increased need for revision surgery. Furthermore, patients implanted with MoM hip joints can also suffer from a condition known as “metallosis” – a potentially fatal complication arising from metallic erosion in the joint which induces pain around the joint, pseudotumors, and a noticeable rash.
Recalls and Lawsuits of MoM Hip Replacement Devices
Smith & Nephew Metal-on-Metal and Hip Resurfacing
Smith & Nephew is a United Kingdom-based joint device manufacturer with operations in the United States. In 2017, Smith & Nephew was named a defendant in Multidistrict Litigation (MDL-2775) occurring in federal court in Maryland before Judge Catherine C. Blake. At the center of the litigation naming Smith & Nephew are several hundred claims alleging defects involving the company’s MoM Birmingham Hip Resurfacing (BHR) implants and its R3 hip joint products. The BHR was the subject of a recall for all units implanted between 2006 and 2015 due to high rates of failure and revision surgeries as was the R3 in 2012 due to issues with erosion and metallosis.
Stryker LFIT V40 Femoral Head and Rejuvenate/ABG II Hip Implant
Stryker is a Michigan-based medical device manufacturer that traces its founding back to Dr. Homer Stryker, an orthopedic surgeon, and his device inventions dating back to 1941. Today Stryker is an international giant that claims $14.9 billion in sales in 2019. In 2016, Stryker announced a recall of its LFIT V40 femoral heads manufactured before 2011 following post-market surveillance showing a higher-than-average rate of failure with symptoms in patients similar to those suffering from metallosis. Similarly, Stryker recalled its Rejuvenate and ABG II implants in 2012 (after only 2 years on the market due to post-market research indicating issues common to MoM devices. Both the LFIT V40 and the Rejuvenate/ABG II devices are the subject of ongoing multi-district litigation in federal court in Massachusetts (MDL-2768) and Minnesota (MDL-2441).
DePuy Orthopedics ASR Hip Implant and Pinnacle Hip Implant
DePuy Synthes is a subsidiary of global pharmaceutical and medical device manufacturer Johnson & Johnson. In 2010, DePuy Synthes issued a voluntary global recall for its ASR Acetabular System following evidence that emerged through post-market surveillance that patients were suffering considerable pain, immune system reactions, inflammation, and the need for revision. The symptoms were consistent with MoM injury and potential design flaws. Similarly, Depuy Synthes’ Pinnacle MoM hip implant is alleged to cause a condition called “osteolysis” which occurs when bone surrounding the hip joint dissolves due to instability and metallosis. Pinnacle has not been the subject of a recall as of this date. However, both the ASR Hip Implant and the Pinnacle Hip Implant are subjects of multi-district litigation in Ohio (MDL-2197) and Texas (MDL-2244).
Zimmer M/L Taper Hip Prosthesis and Versys Femoral Head (MDL-2859)
Indiana-based Zimmer Biomet Holdings, Inc. developed and marketed the Zimmer M/L Taper hip implant with its proprietary Kinectiv Technology. This model of the M/L Taper with the Kinectiv technology utilized a modular MoM design that featured a cobalt-chromium coupling. In court documents, claimants allege that the Zimmer hip joint suffers from corrosion which introduces metal fragments into surrounding tissue as well as elevated levels of metals into the bloodstream causing pain, swelling, tissue necrosis, and other ailments.
Sources Cited (20)
1) “Hip Revision” https://my.clevelandclinic.org/health/treatments/17104-hip-revision
2) “How Long Does It Take to Recover from Hip Replacement Surgery” https://www.thedacare.org/News-and-Events/Symptoms-and-Conditions/How-Long-Does-it-Take-to-Recover-from-Hip-Replacement-Surgery.aspx
3) “About Hip Revision Surgery” https://www.cedars-sinai.org/health-library/diseases-and-conditions/r/revision-hip-surgery.html
4) “Osteolysis” https://www.hss.edu/condition-list_osteolysis.asp
5) “Revision Total Hip Replacement: An Overview” https://www.hss.edu/conditions_revision-total-hip-replacement-overview.asp
6) “Revision surgery of metal-on-metal hip arthroplasties for adverse reactions to metal debris” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6055775/
7) “When to Have Hip Revision Surgery Due to Metal-on-Metal Prostheses” https://holycrossleonecenter.com/when-to-have-hip-revision-surgery-due-to-metal-on-metal-prostheses/
8) “REVISION HIP REPLACEMENT” https://www.stefankreuzermd.com/revision-hip-replacement.html
9) “Preventing Blood Clots After Hip or Knee Replacement Surgery or Surgery for a Broken Hip” https://www.ncbi.nlm.nih.gov/books/NBK107165/#:~:text=DVT%20is%20the%20most%20common,or%20weeks%20after%20the%20surgery.
10) “PREVENTION AND TREATMENT OF BLOOD CLOTS AFTER HIP AND KNEE REPLACEMENT SURGERY” https://www.stoptheclot.org/about-clots/toolkit-for-knee-hip-replacement-patients/prevention-and-treatment-of-blood-clots-after-hip-and-knee-replacement-surgery/
11) “Deep Vein Thrombosis” http://www.ucihealth.org/medical-services/orthopaedics/hip-knee-surgery-services/deep-vein-thrombosis
12) “BLOOD CLOTS IN ORTHOPEDIC SURGERY FACT SHEET” https://www.stoptheclot.org/about-clots/toolkit-for-knee-hip-replacement-patients/orthopedic-surgery-fact-sheet/
13) “Blood Clots After Surgery” https://www.webmd.com/dvt/blood-clots-after-surgery
14) “Hip Dislocation” https://www.hss.edu/condition-list_hip-dislocation.asp
15) “Dislocation Following Total Hip Replacement” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4298240/
16) “Risk factors for dislocation after primary total hip replacement: a systematic review and meta-analysis of 125 studies involving approximately five million hip replacements” https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(19)30045-1/fulltext
17) “Hip replacement” https://www.mayoclinic.org/tests-procedures/hip-replacement/about/pac-20385042
18) “Total Hip Replacement Fundamentals” https://my.clevelandclinic.org/health/treatments/17102-hip-replacement
19) “Signs & Symptoms” https://www.sahortho.com/joint/signs-symptoms#:~:text=Thigh%20or%20groin%20pain%20is,a%20sign%20of%20implant%20loosening.
20) “Hip Replacement Loosening Symptoms” https://www.verywellhealth.com/hip-replacement-loosening-2548623#:~:text=The%20most%20common%20cause%20of,This%20problem%20is%20called%20osteolysis.