Last Updated November 15, 2020
Metallosis is a condition where human tissue and bodily systems are damaged/degraded by contamination from metallic corrosion and the release of toxic metallic debris into the bloodstream. Tissue impacted by metallic corrosion and leaching suffer pain and appear discolored due to “Adverse Local Tissue Reaction” (ALTR) or “Adverse Reaction to Metal Debris” (ARMD).
Depending upon the nature of the metal-alloys present (and other factors), corrosion and leaching can develop into metallosis and more potentially painful joint symptoms as well as dermatologic conditions, depression, dementia, renal failure, cardiovascular issues, and pseudotumors.
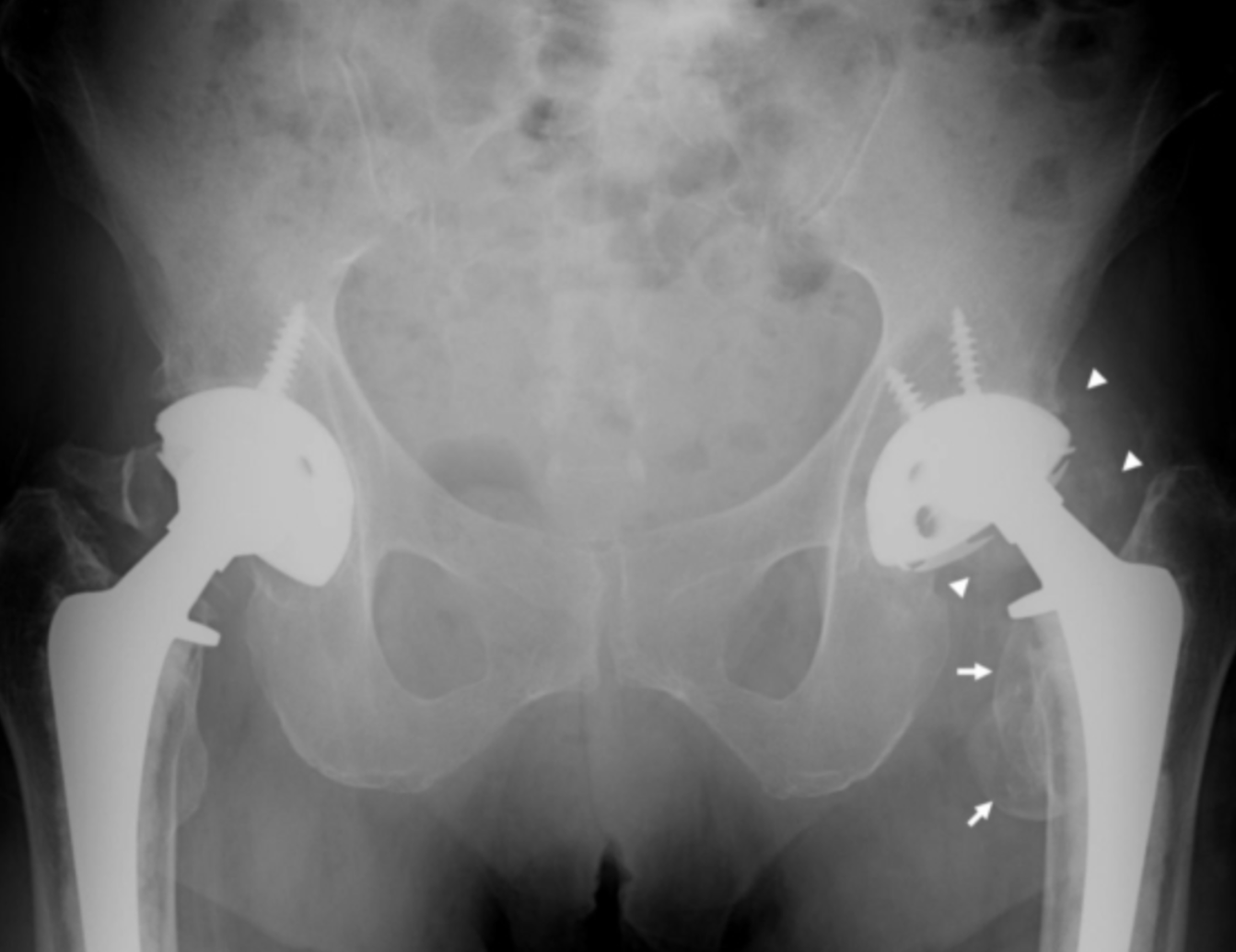
Metallosis has been found to occur as a side effect of hip joints composed of the metallic femoral stem and head combinations – also known as “metal-on-metal” (MoM). In years past, titanium and stainless steel were prevalent in many lines of implantable hip joints. However, in recent years cobalt-chromium combinations emerged as the most desirable for metal-on-metal (MoM) hip implants.
Metal-on-Metal Hip Implants
Beginning in the early 2000s, manufacturers like DePuy, Zimmer, Stryker, and Smith & Nephew all aggressively brought MoM hip joint lines to market through the U.S. Food and Drug Administration’s (FDA) abbreviated 510(k) clearance process. This “fast-track” review allowed manufacturers to bring new MoM hip joints to the market rapidly without having to engage in clinical trials or extensive testing.
MoM hip implants are characterized by a metallic ball and cup pairing which slide against each other when the joint functions (i.e. activities such as walking or running). When the two metal couplings rub each other, metal can begin to shed from the device causing particles to embed in surrounding tissue as well as metallic ions to enter the bloodstream and disperse throughout the body. These particles and ions can progress to metallosis.
As far back as the 1970s, surgeons have recognized the heightened association between MoM and metallosis. Dr. George McKee, a surgeon in the United Kingdom, first reported metallosis in patients who developed painful symptoms 3-4 years following hip replacement with a MoM joint. Dr. McKee’s patients reported progressive pain and instability and upon examination, showed soft tissue that appeared stained green with a grey paste accompanied by bone loss. Large amounts of joint fluid were also reported – either rust, green, cloudy yellow, or grey-colored in appearance.
In May 2016, the FDA shifted its approach to MoM hip joints, requiring all manufacturers to immediately cease and desist from marketing MoM devices until their safety and efficacy could be demonstrably proven with valid supporting scientific evidence. Since that time, no MOM hip replacement devices have been approved for the market in the United States.
Cobalt-Chromium Hip Implants
Cobalt chromium was first used for hip arthroplasty in 1938 by Dr. Marion Smith-Peterson. Dr. Smith-Peterson found that the cobalt-chromium alloy was preferable to others due to its relatively inert nature. As a general rule, any metal alloy implanted into the human body will undergo ionization and start the process of corrosion. However, cobalt-chromium showed itself to be more corrosion-resistant than others.
The same wear and tear associated with other MoM devices can also be a source of cobalt poisoning or acute cobalt toxicity. In addition to other symptoms associated with metallosis, cobalt toxicity can also cause patients to suffer from fevers, inflammation, low thyroid levels, loss of hearing and vision, organ damage, and even heart failure.
Symptoms of Metallosis
Metallosis symptoms typically do not emerge right after hip replacement. They emerge gradually over time. In some cases, metallosis has been reported to manifest anywhere from 3-4 years following surgery. Generally, the symptoms associated with metallosis include:
- Pain around the implant joint
- Pseudo-tumors (nodules resembling tumors – but which are actually fluid collecting)
- Rash
- Osteolysis
- Loosening of the joint
- Raised levels of cobalt and chromium in the blood.
Metallosis and Depression/Dementia
In a 2017 article for BMC Psychiatry, Dr. Ben Green, Dr. Emily Griffiths, and Dr. Solomon Almond examined ten pre-revision surgery patients, nine of whom were suffering from toxic levels of chromium and cobalt in their blood. Among the nine patients, all reported significant levels of depression. Seven of the ten patients had neurocognitive abnormalities, including short-term memory deficits, problems with concentration, and difficulties verbalizing.
Metallosis Treatment Options
Generally speaking, the only option for MoM hip implant patients suffering from complications from metallosis is revision surgery. During revision surgery, the physician will work to excise affected bone and soft tissue around the joint. For more severe cases of metallosis, a surgeon may also elect to resurface. In all cases, the new joint implanted will feature a new stem, head, and acetabular combo likely with either a ceramic or plastic component. The good news for patients is that once the revision is complete, they usually recover quickly.
Sources Cited (23)
1) “Metallosis of the Resurfaced Hip” http://www.pritchettorthopedics.com/articles/pritchett_metallosis_of_the_hip.pdf
2) “Metallosis: A New Form of Autoimmune/Autoinflammatory Syndrome Induced by Adjuvants Syndrome (ASIA)?” https://www.ejcrim.com/index.php/EJCRIM/article/view/1034/1634
3) “Cobalt” https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=19&contentid=Cobalt
4) “Cobalt Toxicity: The Poison in Her Hip Replacement” https://medtruth.com/articles/patient-stories/cobalt-toxicity-the-poison-in-her-hips/
5) “Neuropsychiatric symptoms following metal-on-metal implant failure with cobalt and chromium toxicity” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5259873/
6) “Cancer incidence and cause-specific mortality in patients with metal-on-metal hip replacements in Finland” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3940989/
7) “Can Your Hip Replacement Kill You?” https://www.nytimes.com/2018/01/13/opinion/sunday/can-your-hip-replacement-kill-you.html
8) “Metallosis and Metal-Induced Synovitis Following Total Knee Arthroplasty: Review of Radiographic and CT Findings” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3303397/
9) “The History Of Hip Replacement Surgery” https://www.sonoranhipcenter.com/432-2/
10) “Cobalt poisoning” https://www.mountsinai.org/health-library/poison/cobalt-poisoning
11) “Cobalt Toxicity and Other Metal Hip Replacement Side Effects” https://www.findlaw.com/injury/product-liability/cobalt-toxicity-and-other-metal-hip-replacement-side-effects.html
12) “Revision surgery of metal-on-metal hip arthroplasties for adverse reactions to metal debris” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6055775/
13) “Metallosis after metal-on-polyethylene total hip arthroplasty” https://www.researchgate.net/publication/5452207_Metallosis_after_metal-on-polyethylene_total_hip_arthroplasty
14) “Failure Risks of Metal-on-Metal Hip Implants” http://blog.arthritis.org/news/metal-on-metal-hip-implants-failure-risks/#:~:text=Bone%20loss%20and%20subsequent%20implant,ability%20to%20form%20new%20bone.
15) “Metal-on-Metal Hip Implants” https://www.fda.gov/medical-devices/implants-and-prosthetics/metal-metal-hip-implants
16) “Metal-on-Metal Hip Arthroplasty: A Review of Adverse Reactions and Patient Management” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4598667/
17) “Hip Revision” https://my.clevelandclinic.org/health/treatments/17104-hip-revision#:~:text=Hip%20revision%20surgery%20is%20performed,hip%20can%20function%20normally%20again.
18) “Metallosis: A diagnosis not only in patients with metal-on-metal prostheses” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4750564/
19) “Concerns about Metal-on-Metal Hip Implants” https://www.fda.gov/medical-devices/metal-metal-hip-implants/concerns-about-metal-metal-hip-implants
20) “NIH researchers uncover clues related to metal-on-metal hip implants” https://www.nih.gov/news-events/news-releases/nih-researchers-uncover-clues-related-metal-metal-hip-implants
21) “Metal-on-metal hip replacements: implications for general practice” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5697529/
22) “What is appropriate surveillance for metal-on-metal hip arthroplasty patients?” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5810829/
23) “Metal ion levels comparison: Metal-on-metal hip resurfacing vs total hip arthroplasty in patients requiring revision surgery” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6180337/