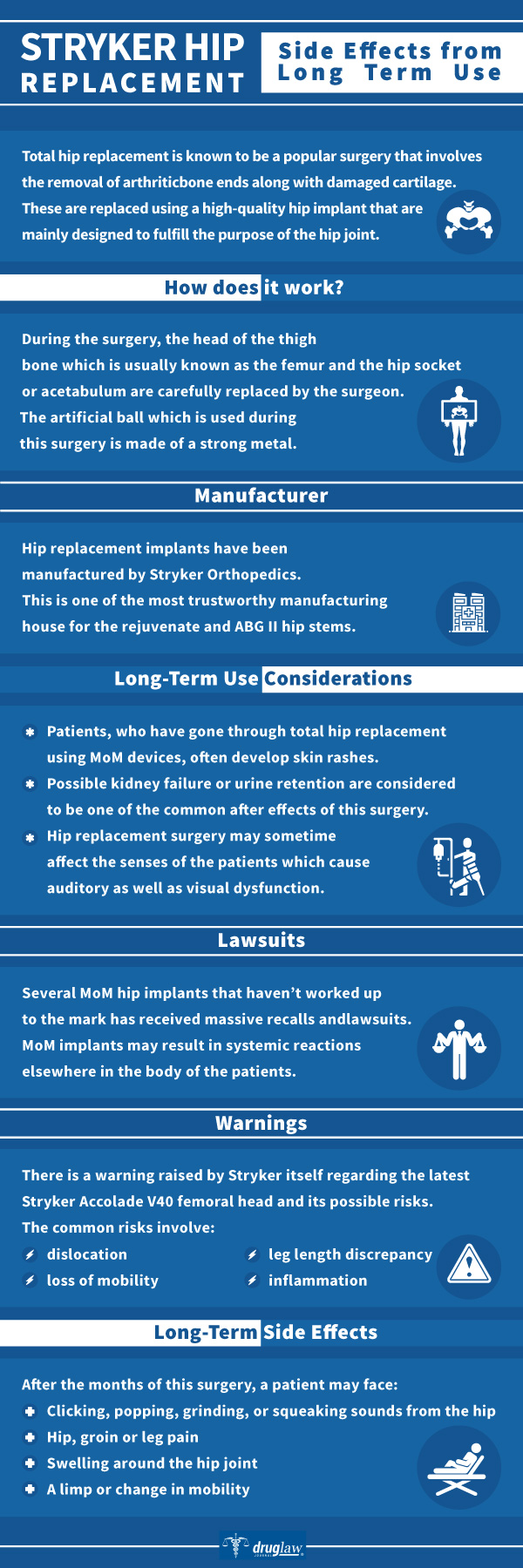
The Stryker Corporation is a well-established and well-known manufacturer of a range of medical devices on the market in the United States today. Over recent decades, the company has positioned itself to be a prominent player in the hip and knee-joint replacement category with several popular joint replacement products. Some have gone on to be huge successes for Stryker, while others, such as the Rejuvenate, ABG II, and LFIT 40 lines became the objects of controversy and later – lawsuits; due to high rates of revision surgery and metal contamination.
History and Background of Stryker
Stryker is a specialty medical equipment manufacturer that designs, develops and manufactures surgical and health care products. From its headquarters in Kalamazoo, Michigan, it has grown to become one of the world’s largest medical technology companies with product lines spanning:
- Orthopaedic Implants
- Orthopaedic Trauma Systems
- Endoscopic Systems
- Patient Care Technologies
Stryker was established in 1941 by Dr. Homer Stryker, an orthopaedic surgeon from Kalamazoo who was dissatisfied with the medical products he encountered in those days. Immediately, he and his company set out to design newer and improved devices to replace the ones that he deemed were ineffective or inefficient. His first successful device was the “Stryker Frame” – a mobile hospital bed which pivoted easily. A common sight in hospitals even today, Stryker beds allow doctors to shift injured patients while keeping them immobilized.
Under the tenure and watch of Dr. Stryker and his son, Lee, Stryker grew steadily through the 1950s and 1960s. However, it wasn’t until the 1970s and the arrival of Stryker’s new CEO, John Brown (formerly of Bristol Myers Squibb) that the company launched down the path of explosive growth that characterizes Stryker to this very day. Over the 32-year span of John Brown’s career at Stryker, he took the company public (1979) and turned Stryker into a perennial favorite of the Fortune 500.
Today, Stryker sells its products in over 100 countries, owns nearly 5,000 patents globally, and has 33,000 employees worldwide. In 2015 it boasted 36 years of straight sales growth and $9.9 billion in sales revenue. Hip implants from its Howmedica Osteonics division, which it purchased from Pfizer Inc., in 1998 for $1.9 billion in cash, accounted for 13% of sales in 2015 alone. Orthopedic implants account for 43% of Stryker’s sales and it maintains a position within the top three manufacturers globally for knee and hip implants – a 22% market share for these devices.
Issues with Stryker Hip Implant Technologies and Product Lines
Accolade, ABG II and Rejuvenate Systems
The Accolade, ABG II and Rejuvenate hip joint systems were all designed and manufactured by Stryker’s Howmedica Osteonics business unit to utilize a proprietary alloy known as “TMZF” (Titanium/Molybdenum/Zirconium/Iron) which, it was believed, would better approximate the elastic properties of bone, while allowing for increased flexibility and strength.
The Accolade system was presented and marketed as a total hip femoral stem for coupling with Stryker’s other proprietary femoral head technologies. Alternatively, the Rejuvenate and ABG II were marketed as femoral stem “modular” component systems with varying sizes of stems (right and left) as well as modular necks. Both sets of device lines utilized TMZF in the stem while ABG II also incorporated a cobalt-chromium alloy into the neck.
The U.S. Food and Drug Administration (FDA) approved the Rejuvenate and ABG II lines for sale in the United States in 2008 and 2009 respectively. Both devices were reviewed under the FDA’s 510(k) clearance process without requiring any extensive studies, clinical trials or pre-market testing prior to implantation in patients. Almost immediately, surgeons and patients started noticing issues with the devices associated with corrosion and fretting at the modular neck-joint. Neck failure and corrosion frequently necessitated expensive and painful revision surgeries and were accompanied by: metallosis; bone necrosis; pseudotumors and acute metal toxicity in the blood.
Both the Rejuvenate and ABG II product lines were recalled in July 2012. Subsequently, Stryker completely eliminated the TMZF alloy from its entire product lineup.
LFIT V40 Femoral Heads
The LFIT V40 femoral head is the “ball” component which was configured more broadly into the overall Stryker TMZF “stem” product line beginning in the early 2000s. Specifically, the femoral head fits neatly into the bone socket of the pelvis and allows freedom of rotation. These devices were marketed ostensibly as a way of minimizing dislocation issues for patients.
In 2016, an investigative report issued by a prominent medical journal concerning issues involving metallic debris and damage to soft tissue stemming from corrosion and wear on the LFIT V40. Not long afterward, Stryker released an urgent “medical device notification” to the surgical community, then recalled nearly 42,000 units of the LFIT V40 due to concerns over the potential for hip dislocation and metallosis.
Tritanium Acetabular Shell
In 2008, Stryker introduced a porous 3D-printed acetabular cup made from Tritanium alloy marked under its “Trident” line. It later introduced its “next-generation” version of the Trident II cup in 2018. The design of the Trident line allows for large femoral head size options and a greater range of motion. According to Stryker, the Trident promises greater joint stability and lower risk of dislocation, while the porous surface is intended to mimic the characteristics of pelvic cancellous bone.
Stryker itself funded a 2013 follow-up study which reviewed the Total Hip Arthroplasties (THA) of 252 patients who received the Tritanium cup. The study, which was conducted, in part, by authors who were receiving royalties from Stryker or had preexisting financial relationships with Stryker, triumphantly reported that 100% of the implants were still successful 25-56 months following surgery.
However, independent studies published between 2017 and late 2018 have uncovered concerns about the safety and effectiveness of the primary Tritanium acetabular cups. Indeed, a study conducted at the NYU Langone Hospital, which was published in 2018, reported that five revision patients who were implanted with the primary Tritanium acetabular cup had failed to achieve “bone-in growth” following THA procedures between 2011 and 2016. At least two other contemporary studies have uncovered similar findings.
What these studies implicate in the primary Tritanium acetabular cup is a “loosening” because of its failure to encourage bone growth following implantation. This bone growth is essential to keeping the cup in place following THA. Consequently, patients loosening may experience pain and swelling; or worse yet – instability (“hip-locking”) or even dislocation.
Sources Cited (21)
1) “Stryker: Our History” https://www.stryker.com/us/en/about/history.html#nineteenthirty
2) “Stryker Fact Sheet” https://www.stryker.com/content/dam/stryker/about/about_landing/pdfs/2016_Fact_Sheet.pdf
3) “Stryker Corporation Backgrounder/Reuters” https://www.reuters.com/companies/SYK
4) “Stryker Corporation History” http://www.fundinguniverse.com/company-histories/stryker-corporation-history/
5) “Early aseptic loosening of the Tritanium primary acetabular component with screw fixation” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5994600/
6) “Corrosion-wear of β-Ti alloy TMZF (Ti-12Mo-6Zr-2Fe) in simulated body fluid” https://pubmed.ncbi.nlm.nih.gov/27397494/#:~:text=Statement%20of%20significance%3A%20TMZF%20is,modular%20design%20total%20hip%20replacement.
7) “The Accolade TMZF stem fulfils the demands of modern stem design: Minimum 5-year survival in a cohort of 937 patients” https://journals.sagepub.com/doi/full/10.1177/2309499018807747
8) “ABG II Modular Anatomic Reconstruction” https://www.hpcbd.com/ABG-II-Modular-Rationale-and-Surgical-Technique.pdf
9) “Stryker Orthopaedics Launches the Rejuvenate Modular Primary Hip System” https://www.prnewswire.com/news-releases/stryker-orthopaedics-launches-the-rejuvenate-modular-primary-hip-system-83884277.html
10) “Stryker to Purchase Howmedica” https://www.meddeviceonline.com/doc/stryker-to-purchase-howmedica-0001
11) “Stryker Adds More Units to LFIT V40 Femoral Head Recall” https://newyork.legalexaminer.com/health/medical-devices-implants/stryker-adds-more-units-to-lfit-v40-femoral-head-recall/
12) “3 RECENT STUDIES QUESTION TRITANIUM ACETABULAR CUP SURVIVAL RATE” https://ryortho.com/breaking/3-recent-studies-question-tritanium-acetabular-cup-survival-rate/3/
13) “Stryker Launches Next Generation Trident® II Acetabular System” https://www.stryker.com/us/en/about/news/2018/stryker-launches-next-generation-trident–ii-acetabular-system.html
14) “Trident® Tritanium™ Acetabular System Surgical Protocol” https://www.strykermeded.com/media/1154/trident-tritanium-acetabular-system-surgical-protocol.pdf
15) “Tritanium Acetabular Cup in Revision Hip Replacement: A Six to Ten Years of Follow-Up Study” https://pubmed.ncbi.nlm.nih.gov/29685709/
16) “Short to Midterm Follow-Up of the Tritanium Primary Acetabular Component: A Cause for Concern” https://pubmed.ncbi.nlm.nih.gov/27642044/
17) “Comparison of a highly porous titanium cup (Tritanium) and a conventional hydroxyapatite-coated porous titanium cup: A retrospective analysis of clinical and radiological outcomes in hip arthroplasty among Japanese patients” https://pubmed.ncbi.nlm.nih.gov/30055877/
18) “Early results of a new highly porous modular acetabular cup in revision arthroplasty” https://pubmed.ncbi.nlm.nih.gov/19876878/
19) “Tritanium acetabular wedge augments: short-term results” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4930514/
20) “Medical Device Maker Accused in Marketing Fraud” https://www.nytimes.com/2009/10/29/business/29device.html
21) “Sales Reps May Be Wearing Out Their Welcome In The Operating Room” https://www.npr.org/sections/health-shots/2018/11/23/659816082/sales-reps-may-be-wearing-out-their-welcome-in-the-operating-room



