Last Updated November 5, 2020
In the United States, the number of Total Hip Arthroplasty (THA) procedures performed in recent years has increased substantially – especially in a growing number of young patients. Physicians and clinicians believe hip replacement surgery is one of the most cost-effective ways to improve the quality of life in suffering patients. However, although there are generally many benefits to suffering patients from hip replacement surgery – as with any invasive procedure, there are risks and the possibility for complications.
The vast majority of patients who undergo THA find significant pain relief and improvement in mobility following the procedure. Bearing that in mind, there are factors such as patient obesity or other medical conditions such as diabetes, liver disease, heart disease, or other uncemented prostheses which may contribute negatively to the postoperative outcome. Patients are always recommended to develop an open line of communication with their physicians and surgeons to identify specific risks prior to surgery and possibly undertake lifestyle modifications such as quitting smoking or losing weight.
The bottom line for both doctors and patients is to avoid unfortunate complications which can have life-altering consequences as well as the need for follow-up surgical procedures known as “revision” surgery. Below are some of the more significant and unwelcome risks associated with hip replacement surgery:
Blood Clots
Deep Vein Thrombosis (DVT) is a widely recognized risk factor following hip replacement surgery. DVT is a blood clot in the leg that can break loose and become a Pulmonary Embolism (PE) that travels through the body and into the lungs where it can cause illness and death. DVT can occur in any vein in the body, however, they tend to manifest the most often in the lower extremities of the body.
Symptoms of DVT may not be obvious or even recognizable to the patient. In fact, DVTs can occur with no symptoms at all about half of the time. When there are visible symptoms, they usually look/feel like:
- Swelling in the leg
- Red, discolored, or white skin
- A cord in a leg vein that can be felt
- Rapid heartbeat
- Slight fever
- Warm skin
- More visible surface veins
- A dull ache, tightness, tenderness, or pain in the leg
Any patient with symptoms that concern them should call their physician immediately.
Risk factors for DVT or that may contribute to DVT include obesity, inherited traits that cause clotting, being older than 60, and having Type-A blood.
Patients can take steps to prevent DVT following surgery as well and physicians may prescribe exercise/physical therapy, compression stockings, and anti-clotting medicines to help combat the risk.
Hip Dislocation
Traumatic hip dislocation occurs when the ball of the hip joint is pushed out of the socket and is usually the result of an incident such as an auto collision or a high-impact fall. A dislocated hip can result in life-changing and long-term issues that can be debilitating if not addressed promptly.
Hip dislocation following THA is relatively infrequent among healthy people under the close guidance and care of a physician. However, there tend to be higher rates of dislocation among some sets of hip replacement patients, such as the elderly or those who have suffered from previous hip injuries.
If a patient or physician suspects that a hip joint has dislocated, they will typically conduct a physical exam and order imaging of the hip with either a CT scan or MRI. An orthopaedist usually can manipulate the hip-joint back into place while the patient is under anesthesia. However, if the damage appears to be more severe, revision surgery may be necessary.
Infection
Infection following surgery for THA is a possibility and a very serious problem. Accidental exposure to bacteria such as staphylococcus aureus can occur even in the midst of the most stringent sterilization protocols.
People who live with certain conditions, such as diabetes mellitus; obesity; peripheral vascular disease; or are otherwise immunocompromised; are at greater risk for developing infections even in a hospital setting.
Common symptoms of joint-infection following surgery include:
- Increased stiffness and pain
- Swelling
- Redness
- Wound drainage
- Fevers, nightsweats and chills
- Fatigue
Treatment options for joint-infection depend upon the extent and depth of the infection. Superficial infections in soft tissue are relatively easy to treat with oral antibiotics or even intravenous (IV) antibiotics. For deeper infections, including those within the joint itself, treatment may require surgical treatments such as debridement – or removal of the infected soft tissue and thorough cleaning of implanted devices and spacers.
Damage to Joint Structures
There exists a distinct possibility that soft muscle and joint tissue surrounding the hip will be damaged during surgery. This damage, in some cases, can result in leg weakness or decreased feeling in the leg.
Loosening of the Prosthetic
A new hip joint should fit naturally with the existing bone and surrounding tissue. However, over time, one or both ends of the replaced joint may loosen the bond to the natural bone causing pain or other problems.
When a hip replacement is implanted, it is either pressed or cemented into position to fit tightly into the bone of the thigh or pelvis. A hip replacement may start to loosen due to factors involving: weight, age, sex, and activity levels. Statistics have shown that women, people over 60, and those with a Body Mass Index (BMI) under 25 tend to have the least amount of issues with loosening.
The most common issue connected with the loosening of the hip joint is Osteolysis – a condition akin to the bone around the implant seemingly “melting away”. Because of the weakened bone around the hip joint, it begins to come loose and wobble.
Loosening issues may necessitate a repair surgery known as a Hip Revision. Revision surgery may also be recommended if an infection has developed in the tissue surrounding the joint.
Femur Fracture
Bone fractures in combination with prosthetic joint replacements are referred to as “periprosthetic fractures”. They are generally rare but are a very serious complication of hip replacement surgery. These types of fractures usually occur years after surgery and can be challenging to repair due to the age of the patient. They are usually the result of a fall or other similar trauma. Most cases of periprosthetic fractures require surgery to treat.

Metal-on-Metal Implants and Metallosis
In Metal-on-Metal (MoM) hip implants, the metal ball and metal cup slide against each other during walking or running. This friction between the ball and socket can cause the release of metal particles that will wear off of the device and embed in surrounding tissue as well as metal ions of cobalt and chromium entering the bloodstream. Over time, this leaching of metal from the joint into surrounding tissue causes “Adverse Local Tissue Reaction” (ALTR) and/or “Adverse Reaction to Metal Debris” (ARMD). The bottom line for patients implanted with MoM joints, however, is that they suffer damage and pain, device failure, loosening, and the need for revision surgery. Furthermore, patients implanted with MoM hip joints can also suffer from a condition known as “metallosis” – a potentially fatal complication arising from metallic erosion in the joint which induces pain around the joint, pseudotumors, and a noticeable rash.
Prior to 2016, MoM hip devices marketed in the United States were subject to the FDA’s less rigorous 510(k) clearance process which allowed them to be implanted in human patients without first subjecting them to the same standards of scientific review and lab testing as the FDA’s more rigorous Pre-Market Approval (PMA) process. In May 2016, following numerous recommendations, the FDA required all MoM manufacturers to immediately cease and desist from marketing MoM devices until they can be demonstrably approved under PMA conditions with valid supporting scientific evidence. It is worth noting that to-date, there are no FDA-approved MoM hip replacement devices marketed for use in the United States.
Hip Revision
Despite the overall favorable statistics concerning THA, hip replacement surgery can still fail for a number of reasons. When hip replacement failure occurs, a physician may recommend a second procedure to examine or even remove some of a patient’s prosthesis and replace it with a new device. This second procedure is known as a “revision”.
The hip revision could turn out to be a longer and more complex procedure than even the first surgery. Additionally, there are different types of hip revision surgery depending upon the extent of injury or replacement. In some cases, only parts of a prosthetic joint may be addressed, while in another revision – the entire joint may be replaced.
As discussed earlier, a physician may order a revision on the basis of a range of circumstances, including infection; fracture; loosening; dislocation, or reaction to metallosis. Revision surgery carries similar risks as primary hip replacement surgery. However, the success rate for revision surgery is generally lower due to issues with the structural strength of the bone.
Patients undergoing revision surgery can take certain steps to minimize risks in advance, such as:
- Avoiding overuse of the joint
- Avoiding sports which involve jumping
- Maintaining a healthy weight
Following revision surgery, patients should keep in close contact with their physician and surgeon if additional pain develops suddenly or it appears that an infection has developed.
Sources Cited (43)
1) “Complications of Total Hip Arthroplasty: Standardized List, Definitions, and Stratification Developed by The Hip Society” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4709292/#:~:text=Based%20on%20this%20work%20from,instability%2C%20periprosthetic%20fracture%2C%20abductor%20muscle
2) “Complications Following Total Hip Arthroplasty” https://www.intechopen.com/books/arthroplasty-update/complications-following-total-hip-arthroplasty
3) “Complications of total hip arthroplasty” https://www.uptodate.com/contents/complications-of-total-hip-arthroplasty
4) “THA Other Complications” https://www.orthobullets.com/recon/5030/tha-other-complications
5) “Causes of Painful THA” https://hipandkneebook.com/complications
6) “Periprosthetic joint infection: Current concept” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3601222/
7) “Joint Replacement Infection” https://orthoinfo.aaos.org/en/diseases–conditions/joint-replacement-infection/
8) “Preventing Blood Clots After Hip or Knee Replacement Surgery or Surgery for a Broken Hip” https://www.ncbi.nlm.nih.gov/books/NBK107165/#:~:text=DVT%20is%20the%20most%20common,or%20weeks%20after%20the%20surgery.
9) “PREVENTION AND TREATMENT OF BLOOD CLOTS AFTER HIP AND KNEE REPLACEMENT SURGERY” https://www.stoptheclot.org/about-clots/toolkit-for-knee-hip-replacement-patients/prevention-and-treatment-of-blood-clots-after-hip-and-knee-replacement-surgery/
10) “Deep Vein Thrombosis” http://www.ucihealth.org/medical-services/orthopaedics/hip-knee-surgery-services/deep-vein-thrombosis
11) “BLOOD CLOTS IN ORTHOPEDIC SURGERY FACT SHEET” https://www.stoptheclot.org/about-clots/toolkit-for-knee-hip-replacement-patients/orthopedic-surgery-fact-sheet/
12) “Blood Clots After Surgery” https://www.webmd.com/dvt/blood-clots-after-surgery
13) “Hip Dislocation” https://www.hss.edu/condition-list_hip-dislocation.asp
14) “Dislocation Following Total Hip Replacement” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4298240/
15) “Risk factors for dislocation after primary total hip replacement: a systematic review and meta-analysis of 125 studies involving approximately five million hip replacements” https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(19)30045-1/fulltext
16) “Hip replacement” https://www.mayoclinic.org/tests-procedures/hip-replacement/about/pac-20385042
17) “Total Hip Replacement Fundamentals” https://my.clevelandclinic.org/health/treatments/17102-hip-replacement
18) “Signs & Symptoms” https://www.sahortho.com/joint/signs-symptoms#:~:text=Thigh%20or%20groin%20pain%20is,a%20sign%20of%20implant%20loosening.
19) “Hip Replacement Loosening Symptoms” https://www.verywellhealth.com/hip-replacement-loosening-2548623#:~:text=The%20most%20common%20cause%20of,This%20problem%20is%20called%20osteolysis.
20) “Revision Total Hip Replacement: An Overview” https://www.hss.edu/conditions_revision-total-hip-replacement-overview.asp
21) “Dislocation after THA remains a dreaded complication for patients and surgeons” https://www.healio.com/news/orthopedics/20160525/dislocation-after-tha-remains-a-dreaded-complication-for-patients-and-surgeons
22) “Fracture After Total Hip Replacement” http://www.ucihealth.org/medical-services/orthopaedics/hip-knee-surgery-services/fracture-total-hip-replacement
23) “Periprosthetic Fractures of the Femur After Hip Arthroplasty: An Analysis of 99 Patients” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2504263/
24) “Fractures around a hip replacement or knee replacement” https://uihc.org/health-topics/fractures-around-hip-replacement-or-knee-replacement
25) “Total hip arthroplasty.” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1022709/
26) “Management of the Infected Total Hip Arthroplasty” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5525520/
27) “Hospitalization for Total Hip Replacement Among Inpatients Aged 45 and Over: United States, 2000–2010” https://www.cdc.gov/nchs/products/databriefs/db186.htm
28) “Prevalence of Total Hip and Knee Replacement in the United States” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4551172/
29) “Metal-on-Metal Hip Implants” https://www.fda.gov/medical-devices/implants-and-prosthetics/metal-metal-hip-implants#:~:text=To%20date%2C%20there%20are%20no,for%20use%20in%20the%20US.&text=Some%20patients%20who%20had%20a,%2Don%2Dmetal%20hip%20implant.
30) “Metal-on-Metal Hip Implants: The FDA’s Activities” https://www.fda.gov/medical-devices/metal-metal-hip-implants/metal-metal-hip-implants-fdas-activities
31) “Effectiveness of Metal-on-Metal Hip Implants” https://www.fda.gov/medical-devices/metal-metal-hip-implants/effectiveness-metal-metal-hip-implants
32) “Information about Soft Tissue Imaging and Metal Ion Testing” https://www.fda.gov/medical-devices/metal-metal-hip-implants/information-about-soft-tissue-imaging-and-metal-ion-testing
33) “Revision Total Hip Replacement” https://orthoinfo.aaos.org/en/treatment/revision-total-hip-replacement/
34) “Hip Revision Surgery Fundamentals” https://my.clevelandclinic.org/health/treatments/17104-hip-revision
35) “Product Classification” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPCD/classification.cfm?ID=LPH
36) “Hip Revision” https://www.hss.edu/condition-list_hip-revision.asp
37) “About Revision Hip Surgery” https://www.cedars-sinai.org/health-library/diseases-and-conditions/r/revision-hip-surgery.html
38) “What patients need to know about revision surgery after hip or knee replacement” https://www.sciencedaily.com/releases/2013/12/131231132730.htm
39) “Total Hip Arthroplasty: Past, Present, and Future. What Has Been Achieved?” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6892902/
40) “A look ahead to the future of hip replacement and hip resurfacing” http://www.opnews.com/2019/01/future-of-hip-replacement-and-hip-resurfacing-article/15075
41) “COMPLICATIONS OF HIP & KNEE REPLACEMENT” http://newyorkorthopedics.com/ny-orthopedics-doctors-highlights/complications-of-hip-knee-replacement-surgery-and-revision-surgery-options/
42) “Joint Replacement Surgery & Infection Treatment” https://www.newyorkhipknee.com/conditions/joint-infection/
43) “Complications of Hip Replacement Surgery” https://www.verywellhealth.com/problems-with-hip-replacement-surgery-2549564
Hernia Mesh Medical Issues and Complications
Last Updated November 4, 2020
Surgical mesh devices used to repair hernias have been available for over a century in the United States and hernia repair is one of the most common surgical procedures performed today. In fact, more than 80% of hernia repairs performed in the U.S. use mesh, and, at the moment, there are more than 70 types of meshes commercially available.
It goes without saying that the hernia mesh industry is both profitable and competitive and manufacturers are incentivized to bring as many “new and improved” products to market as soon as possible. Most new hernia mesh brands are pushed through the U.S. Food and Drug Administration’s (FDA) 510(k) clearance or “fast track” process with little testing or laboratory review. Accordingly, hernia mesh devices sometimes present serious medical issues and complications for the patients into whom they are implanted.
Hernia Mesh Adhesion
Adhesions are bands of fibrous scar tissue that sometimes form following abdominal surgery. In some instances, these scars will form tissue that “sticks” or binds together inside of the body. Adhesions make normally slippery internal tissues collect together and can pull on small or large intestines causing intense pain. This condition can also form in people who develop peritonitis, an infection that spreads to the membrane covering the abdominal organs.
In cases where adhesive tissue becomes severe, bowel obstruction can result and cause cramp-like pains as well as other symptoms such as:
- Abdominal pain
- Nausea and vomiting
- Abdominal swelling
- Inability to pass gas and/or infrequent bowel movements
- Dehydration (dry skin, severe thirst, infrequent urination, swollen/dry tongue)
In cases where adhesive bowel obstruction is partial, surgery may be avoided with rehydration and insertion of a small suction tube through the nose into the stomach to prevent additional bloating. Complete bowel obstruction caused by adhesion often requires surgery to restore blood flow.
Hernia Mesh Contraction
Mesh contraction (sometimes referred to as “mesh shrinkage”) is a phenomenon that, when it occurs, usually does so within two months of being implanted in the body. It is usually characterized by a failure of the implanted mesh to adequately meld with surrounding tissue which results in the mesh no longer covering the hernia location. This often leads to the recurrence of the hernia and further surgery to retrieve the defective mesh and repair the hernia.
Hernia Mesh Migration
Mesh migration occurs when a surgically implanted mesh (typically through laparoscopic implantation) fails to adequately adhere to surrounding tissue and begins to move freely through organ cavities in the abdomen. Migration can result in serious complications for patients, such as erosion into viscous. As a consequence of erosion due to migration, infections and abscesses can form. If treatment for migration and erosion into the bowels is required, it can involve additional procedures such as laparotomy; bowel resection; mesh resection/removal, or anastomosis; followed by another procedure to repair the original hernia.
Hernia Mesh Fistula
Abdominal fistulas can form when a defective hernia mesh fails to properly adhere to surrounding tissue or “erodes” into the gastrointestinal tract. The mesh can cause an abnormal connection between internal organs and the intestines creating fluid leakage into body cavities (or even through the skin). Depending upon the location of the leakage, fistulas may cause diarrhea or poor absorption of nutrients and dehydration. Other fistulas may manifest no symptoms at all and even close on their own after a few weeks or months.
Physicians will ordinarily seek to locate a fistula using barium tracers, CT scans, and fistulograms to closely examine the stomach, bowels, or other areas of the abdomen. Treatment for fistulas may involve antibiotics; immunosuppressants; surgery or intravenous nutritional supplements.
Bowel Perforation Following Hernia Mesh Implantation
Hernia mesh migration and/or erosion can sometimes result in direct trauma to the bowels in the abdomen known as “perforation”. A perforation is a hole that can develop anywhere in your gastrointestinal tract between your throat and your rectum. When that hole or puncture occurs on your large intestine, it is a “bowel perforation”. A perforated bowel can cause the contents of the bowel to spill into your abdomen resulting in an infection known as peritonitis and eventually a more deadly condition known as “sepsis”. Common signs of a perforated bowel are:
- Severe stomach pain
- Chills
- Fever
- Nausea
- Vomiting
Bowel perforations are considered a medical emergency and physicians will seek to treat them immediately by first running X-rays and bloodwork to determine if an infection has developed. Next surgeons will work to repair the perforation. In some cases, the surgeon will perform an ileostomy or colostomy to empty the contents of the bowel while allowing the tissue to heal.
Sources Cited (28)
1) “Is surgical mesh safe for my hernia surgery?” https://www.ucihealth.org/blog/2018/06/mesh-hernia-repair
2) “Two cases about mesh adhesion to intra-abdominal cavity tissue after using mesh to repair an incisional hernia” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5648932/
3) “Degree of Adhesions After Repair of Incisional Hernia” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3041039/
4) “Prevention of adhesions to polypropylene mesh in a traumatized bowel model” https://www.journalacs.org/article/S1072-7515(00)00337-9/fulltext
5) “4 Best Ways to Take Control of Abdominal Adhesions” https://health.clevelandclinic.org/4-best-ways-to-take-control-of-abdominal-adhesions/
6) “Relationship between tissue ingrowth and mesh contraction” https://pubmed.ncbi.nlm.nih.gov/15977079/
7) “Are you aware of these dangerous complications from hernia mesh implants?” https://wsbt.com/sponsored/foley-small/are-you-aware-of-these-dangerous-complications-from-hernia-mesh-implants
8) “Relationship Between Tissue Ingrowth and Mesh Contraction” https://www.researchgate.net/publication/7766806_Relationship_Between_Tissue_Ingrowth_and_Mesh_Contraction
9) “Enterocutaneous fistula due to mesh fixation in the repair of lateral incisional hernia: a case report” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2613897/
10) “Chronic Infection and Enterocutaneous Fistula Secondary to Mesh Migration and Erosion into the Small Bowel” https://www.liebertpub.com/doi/full/10.1089/crsi.2017.0003?mobileUi=0&
11) “Enterocutaneous Fistula” https://herniacenter.ucsf.edu/conditions–procedures/enterocutaneous-fistula.aspx
12) “A Rare Complication of Composite Dual Mesh: Migration and Enterocutaneous Fistula Formation” https://www.hindawi.com/journals/cris/2015/293659/
13) “Mesh fistula after ventral hernia repair: What is the optimal management?” https://www.sciencedirect.com/science/article/abs/pii/S0039606019306786
14) “Management of post-operative complications in open ventral hernia repair” https://parjournal.net/article/view/3272
15) “Enterocutaneous fistula secondary to mesh erosion of bowel: a late complication of polypropylene mesh use in ventral hernia repair” https://www.ijsurgery.com/index.php/isj/article/view/4976
16) “Mesh migration into the sigmoid colon after inguinal hernia repair presenting as a colonic polyp: A case report and review of literature” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6212604/
17) “Mesh migration – Should it be a mandatory consentable complication?” https://www.ijcrisurgery.com/archive/article-full-text/100057Z12PP2019
18) “Asymptomatic migration of ventral mesh for incisional hernia into the small intestine: A case report” https://onlinelibrary.wiley.com/doi/full/10.1002/ccr3.2212
19) “Severe complications after mesh migration following abdominal hernial repair: report of two cases and review of literature.” https://europepmc.org/article/pmc/pmc6545489
20) “Mesh penetrating the cecum and bladder following inguinal hernia surgery: a case report” https://jmedicalcasereports.biomedcentral.com/articles/10.1186/s13256-017-1435-8
21) “Mesh Infection and Migration after Umbilical Hernia Repair” https://www.researchgate.net/publication/276002819_Mesh_Infection_and_Migration_after_Umbilical_Hernia_Repair
22) “Intestinal perforation after surgical treatment for incisional hernia: iatrogenic or idiopathic?” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5917326/
23) “Acute abdomen in the centenary patient, mesh migration into the sigmoid colon after laparoscopic inguinal hernia repair (TAPP): A case report and review of literature” https://www.sciencedirect.com/science/article/pii/S2210261219306844
24) “CT findings of complications after abdominal wall repair with prosthetic mesh” https://www.sciencedirect.com/science/article/pii/S2211568417300037
25) “Long-term Complications Associated With Prosthetic Repair of Incisional Hernias” https://jamanetwork.com/journals/jamasurgery/fullarticle/211564
26) “Abdominal Adhesions” https://www.health.harvard.edu/a_to_z/abdominal-adhesions-a-to-z
27) “Gastrointestinal fistula” https://www.mountsinai.org/health-library/diseases-conditions/gastrointestinal-fistula
28) “Chronic abdominal pain secondary to mesh erosion into cecum following incisional hernia repair: a case report and literature review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959323/#:~:text=Mesh%20migration%20following%20hernia%20repair,are%20the%20most%20common%20sequelae.
Zantac Cancer Risk
Last Updated November 12, 2020
Zantac is the product name for the drug ranitidine. Approved by the U.S. Food and Drug Administration (FDA) in 1984 for the treatment and prevention of ulcers, heartburn, esophagitis, and Zoeller-Ellison syndrome, Zantac quickly rose to become one of the most popular prescriptions and over-the-counter (OTC) H2-blockers on the market. In 2018, Zantac ranked as one of the top-10 antacid tablets in the United States.
In March 2019, laboratories in the United States tested Zantac for the presence of carcinogens and discovered that some forms of Zantac were testing positive for the presence of N-Nitrosodimethylamine, also known as “NDMA” – a potent carcinogen. After further testing and analysis, the FDA ordered all Zantac pulled from shelves in April 2020. Today, more than 500 individuals have filed claims in federal court in Florida alleging that taking Zantac caused their cancer.
NDMA and Zantac Cancer Risk
NDMA is an organic chemical that is both naturally occurring or as a result of industrial processes. NDMA and related compounds can typically be found in tobacco, detergents, and solvents. They may also form in foods such as cured meats as part of a reaction between nitrites and amines. NDMA is rarely ever produced intentionally in the United States unless it is used for laboratory experiments.
NDMA is classified by the International Agency for Research on Cancer (IARC) as Group 2A – meaning they deem it as “probably carcinogenic to humans”. Its harmful and carcinogenic effects are thought to stem from its propensity to cause irreversible DNA mutations and chemical properties which initiate the development of cancers. Animal studies have revealed that NDMA is acutely toxic to rats and even short-to-medium term exposure in other animals has shown damage to multiple organs. In humans, intentionally poisoned with NDMA, medical records demonstrate severe liver damage and internal bleeding.
How Did NDMA Contaminate Zantac?
Researchers are not exactly clear on how they believe NDMA came to be found in Zantac. Its presence is not generally believed to be the result of the manufacturing processes. Valisure, the small Connecticut laboratory which first conducted tests of Zantac for NDMA, has stated that it is likely formed as the result of the inherent instability of ranitidine (the drug molecule at the heart of Zantac). In fact, Valisure believes that the NDMA may occur as a natural byproduct of Zantac breaking down over time.
A 2016 study conducted by Stanford University may support the notion that NDMA’s presence in Zantac is a function of the ranitidine molecule itself. The study gave ten healthy volunteers doses of Zantac and found later that their urine contained trace amounts of NDMA. The presence of NDMA in urine also raises the specter that, if ranitidine breaks down into NDMA, it could also be present in sewage systems or contaminate sources of groundwater.
Which Cancers is NDMA Associated With?
There are numerous causes for cancer ranging from infectious agents; to genetic predisposition; to environmental factors and behaviors such as smoking, alcohol use, diet, obesity, and exposure to pollution. Furthermore, studies and research have yet to draw a conclusive link between NDMA in Zantac and cancer. Accordingly, at this time, it is difficult to calculate the specific cancer risk from NDMA contamination.
With that in mind, researchers have theorized that long-term exposure to NDMA contamination in Zantac could be linked with the following cancers:
- Stomach
- Bladder
- Colorectal
- Liver
- Esophageal
- Small Intestinal
Although less prevalent in research, NDMA has also been linked to these types of cancers:
- Prostate
- Ovarian
- Pancreatic
- Lung
- Uterine
- Brain
- Breast
- Thyroid
- Testicular
- Leukemia
- Non-Hodgkin’s Lymphoma
- Multiple Myeloma
FDA Recall
NDMA contamination in Zantac first came to light following the work of a small laboratory in Connecticut known as Valisure. This laboratory was created after one of its founders suffered from a contaminated batch of medication. Valisure began testing Zantac in March 2019 on the founder’s whim after it was prescribed for his infant daughter to combat acid reflux. The testing immediately revealed abnormal levels of NDMA contamination.
After more testing, Valisure notified the FDA and European drug regulators who each immediately began their own investigations. In October 2019, the FDA ordered all manufacturers of ranitidine products to test for NDMA. That order was followed-up with a broad recall order from the FDA in April 2020, clearing all ranitidine products off the shelves throughout the United States.
Sources Cited (12)
1) “NDMA, a contaminant found in multiple drugs, has industry seeking sources and solutions” https://cen.acs.org/pharmaceuticals/pharmaceutical-chemicals/NDMA-contaminant-found-multiple-drugs/98/i15
2) “The Zantac problem: What’s NDMA?” https://abcnews.go.com/Health/zantac-problem-whats-ndma/story?id=65799147
3) “Ranitidine Cancer Risk“ https://www.medpagetoday.com/meetingcoverage/ddw/86314
4) “Health Risks Associated with N-Nitrosodimethylamine (NDMA)” https://www.labmanager.com/insights/health-risks-associated-with-n-nitrosodimethylamine-ndma-656
5) “What We Know about the Possible Carcinogen Found in Zantac” https://www.scientificamerican.com/article/what-we-know-about-the-possible-carcinogen-found-in-zantac/#:~:text=In%20its%20petition%2C%20Valisure%20also,break%20down%20to%20form%20NDMA.
6) “The Finding of N‐Nitrosodimethylamine in Common Medicines” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7288647/
7) “Oral intake of ranitidine increases urinary excretion of N-nitrosodimethylamine” https://pubmed.ncbi.nlm.nih.gov/26992900/
8) “Formation Mechanism of NDMA from Ranitidine, Trimethylamine, and Other Tertiary Amines during Chloramination: A Computational Study” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4123930/
9) “Statement alerting patients and health care professionals of NDMA found in samples of ranitidine” https://www.fda.gov/news-events/press-announcements/statement-alerting-patients-and-health-care-professionals-ndma-found-samples-ranitidine
10) “Clinical Study to Investigate the Urinary Excretion of N-nitrosodimethylamine (NDMA) After Ranitidine Administration” https://clinicaltrials.gov/ct2/show/NCT04397445
11) “FDA asks manufacturers to remove ranitidine from market: What now?” https://www.aappublications.org/news/2020/07/01/focus-ranitidine070120
12) “Concerned about the ranitidine (brand name: Zantac) recall? Here’s what the FDA says” https://www.miamiherald.com/news/health-care/article236067123.html
E-Cigarettes, Juul and Vaping Lawsuits and Updates
Last Updated October 9, 2020
The e-cigarette devices commonly seen around the United States were invented by a Chinese pharmacist, Hon Lik. In 2003, Hon Lik was issued the very first patent for a device using a lithium battery to heat nicotine into a vaporous mist for inhalation. By 2006, e-cigarettes exploded onto the market in the United States and were immediately touted by their manufacturers as either being less dangerous than combustible smoking or as a valuable tool for smoking cessation. E-cigarette use came to be known colloquially as “vaping” or later “juuling” by both the industry and users.

For at least the first decade in the market, manufacturer promotion of vaping products as “safe” or “safer” products went virtually unchallenged. However, with the passage of time, and upon the initiative of scientific experts within the medical community, more and more evidence has begun to emerge which cuts to the very heart of the manufacturer’s safety claims about vaping and e-cigarette products.
In 2018, the first lawsuits against e-cigarette manufacturers started cropping up in federal and state courts throughout the United States. For one of the most popular manufacturers of e-cigarettes, Juul Labs, Inc. (Juul), the lawsuits stemming from its alleged marketing practices have become so numerous that over 650 actions have been consolidated into one multidistrict litigation in federal court in California.
Background and Legal Implications for Vaping
With some exceptions, lawsuits and investigations connected with vaping and the use of e-cigarettes typically allege that e-cigarette manufacturers (in particular, Juul) have:
- Engaged in marketing practices that target young or underage users.
- Used nicotine salts in their products and failed to warn consumers of the chemical’s presence. Nicotine salts are a form of nicotine that differs from the “freebase” form found typically in e-cigarettes and is believed to be far more addictive.
- Improperly labeled the nicotine dosage in their products.
- Improperly marketed their devices as “safer” than combustible smoking.
- Designed a product with an inherent defect that allows excessive nicotine delivery which causes physical harm and addiction.
Alongside these lawsuits, the vaping industry has attracted increased scrutiny from state and federal regulators concerned with the popularity of vaping devices among younger users.
- In 2018, the U.S. Surgeon General declared e-cigarette use “has become an epidemic among our nation’s young people” and in 2016 the U.S. Food and Drug Administration (FDA) finalized a rule giving the Center for Tobacco Products (CTP) regulatory authority over e-cigarettes.
- In 2020, the FDA used that authority to ban the sale of flavored e-cigarette cartridges (except for menthol and tobacco).
- In September 2019, the U.S. Attorney for the Northern District of California announced a criminal investigation of Juul Labs. A few months later, the attorneys general for 39 U.S. states announced their own investigations into the marketing and sales practices of Juul Labs.
Juul Labs and Big Tobacco
Juul is a brand name for a particular type of e-cigarette that is very popular and accounts for about 40% of all e-cigarette sales in the market. Juuls are shaped like USB flash drives and do not resemble traditional, more bulky e-cigarettes. The cartridge packs that come with Juuls frequently contain more nicotine than actual combustible tobacco cigarettes. Juul has become so popular that kids and teens refer to the practice of vaping with a Juul device as “juuling”.
Juul was established in 2015 as “Juul Labs, Inc.” by two entrepreneurs, Adam Bowen and James Monsees who developed the technology themselves. Manufactured in China, Juul grew dramatically in the intervening years and in July 2018, raised $650 million giving the company a valuation of approximately $15 billion. Later, global tobacco producer, Altria, purchased a 35% interest in Juul making the company worth an estimated $38 billion at the end of 2018.
Alleged Breathing Issues Tied to Vaping
E-Cigarette manufacturers have long pushed the notion that their products are either safe or safer than cigarettes. However, users of e-cigarettes and their families have come forward in recent years telling of horrible suffering that they believe is directly attributable to vaping.
- In September 2019, 18-year old Adam Hergenreder was hospitalized and on respiratory support after two years of steady vaping. Mint and mango were his favorite flavors. Doctors have told Adam that his chest X-rays reveal the lungs of a 70-year old man and that they may never truly heal or be the same again.
- The American Thoracic Society (ATS) published an article on the prevalence of Bronchiolitis Obliterans Organizing Pneumonia (BOOP) in patients claiming they use e-cigarettes. BOOP is a rare inflammatory lung disorder involving flu-like symptoms and coughing that make breathing very difficult in sufferers.
- The December 2019 Mayo Clinic Proceedings examined the potential for breathing issues and vaping in its article: “Vaping-related Acute Lung Injury: A New Killer Around the Block.” Among the article’s highlights was the conclusion that the incidence of vaping-related acute lung injury is increasing and that electronic cigarette compounds present the potential for a range of detrimental effects on the human respiratory system.
- The Journal of the Missouri State Medical Association’s November 2019 edition gave an extensive look into the increasingly bleak outlook for Vaping Associated Lung Injury (EVALI) in the United States. The articles noted that as of its publication, there were 2,051 cases of e-cigarette or vaping lung product uses associated with lung injury (with 39 reported deaths) in the U.S. Overwhelmingly, EVALI patients complain of symptoms like cough, chest pain, and shortness of breath. Of the documented cases expressing EVALI, 86% of the patients reported using THC-containing products. In February 2019, the New England Journal of Medicine and the Centers for Disease Control (CDC) had both established research pieces linking Vitamin E Acetate, a thickening fluid used predominantly in THC vaping products with EVALI.
Legal Exposure for Juul and Other E-Cigarette Manufacturers
State Lawsuits Over Targeting of Children
The New York State Attorney General, Letitia James, announced in November 2019 that her office would lead a lawsuit against Juul Labs for “deceptive and misleading” marketing aimed at children. In her announcement, James referenced Juul “launch parties” which involved direct outreach to high schoolers by Juul reps who repeatedly assured students that Juul was safer than cigarettes. The New York lawsuit mirrored similar lawsuits filed earlier in 2019 by California Attorney General Xavier Becerra and Los Angeles District Attorney Jackie Lacey.
Alleged Hemorrhagic Stroke
Maxwell Berger is a 22-year old Syracuse University student that began using Juul in 2015 and quickly developed a two-pod-per-day habit. In 2017, Maxwell suffered a severe hemorrhagic stroke that paralyzed the left side of his body and caused him to lose half of his vision in both eyes. He has suffered from cognitive impairment and brain damage as well. In 2019, Maxwell sued Juul in New York alleging that his use of Juul caused his stroke and injuries.
Contaminated Juul Pods
In October 2019, a former Juul Labs executive, Siddarth Breja, filed a lawsuit in federal court in California that at least one million contaminated Juul pods were let onto the market by the company despite his warnings to the company’s leadership. Among the claims made by Breja in the case was Juul’s CEO Kevin Burns said: “Half our customers are drunk and vaping like mo-fos, who the f**k is going to notice the quality of our pods.”
Breja’s lawsuit did not specify what type of contamination was alleged to manifest in the pods. Furthermore, Juul’s CEO has publicly denied the allegations and claimed that Juul products are tested for toxicity.
Multidistrict Litigation in California
In 2018, two lawsuits were filed in California alleging that Juul was inappropriately marketed as safe even though it contains more nicotine than a traditional cigarette. Thus began a wave of civil lawsuits naming Juul which have now been consolidated into Multi-District Litigation (MDL) before the federal court in the Northern District of California. As of this moment, MDL-2913 has approximately 651 cases pending and is growing.
FDA Investigation Over Possible Juul LInk to Seizures
Bloomberg News obtained communications and memoranda between FDA officials in October 2018 detailing three cases of seizures alongside significant Juul use. While the FDA documentation did not detail any causality between Juul and the seizures, they believe that at a minimum there was an “association” with Juul. Over the next few months, the FDA uncovered an additional 32 reports of e-cigarette use that were believed to be linked to seizures. The FDA announced an investigation into the link between seizures and vaping in April 2019.
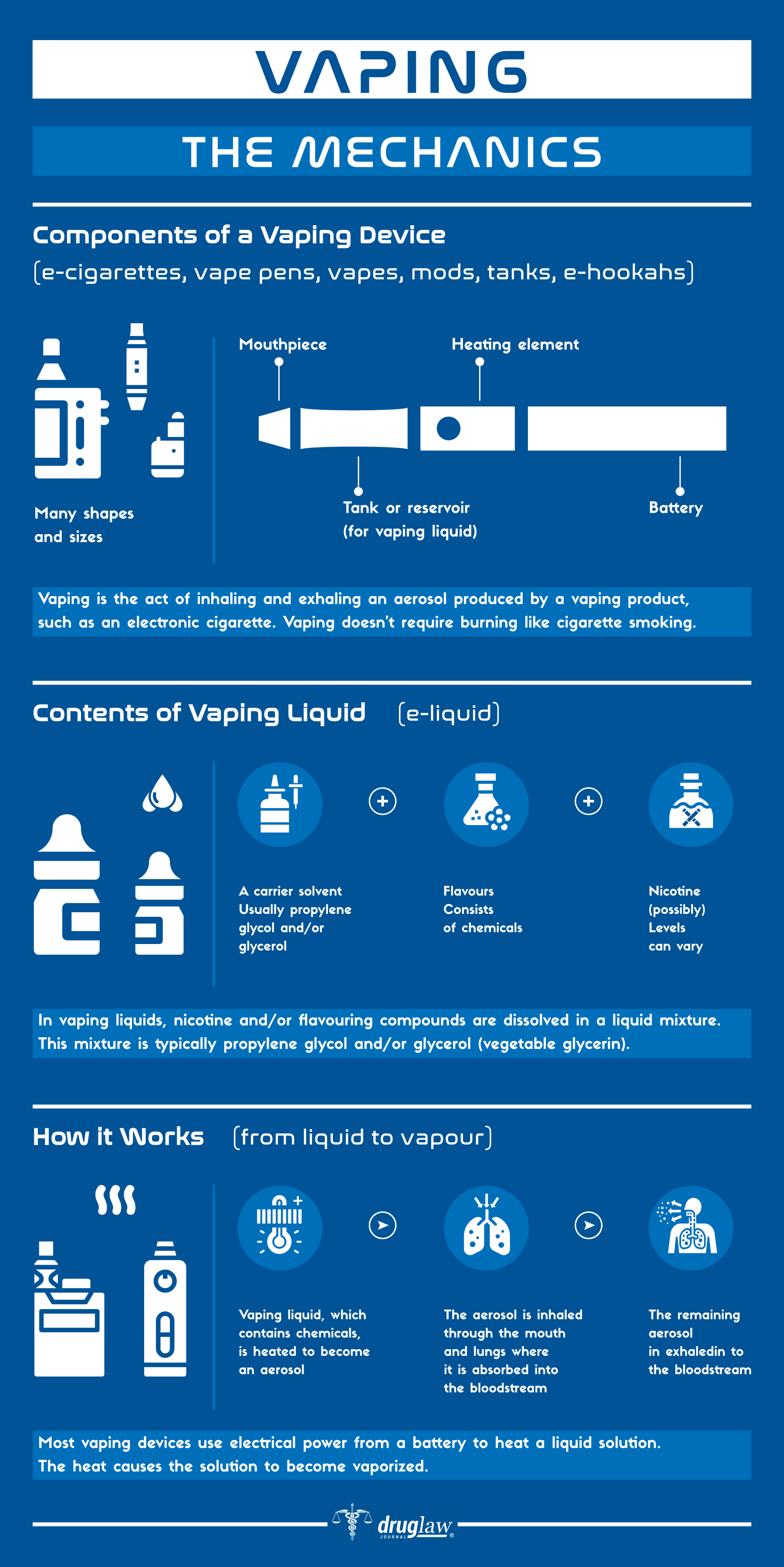
Sources Cited (35)
1. “Juul hit with another state lawsuit for allegedly targeting kids” https://www.nbcnews.com/health/vaping/juul-hit-another-state-lawsuit-allegedly-targeting-kids-n1085901
2. “Public health groups fuming over Trump’s inaction on vaping flavor ban” https://www.nbcnews.com/health/vaping/public-health-groups-fuming-over-trump-s-inaction-vaping-flavor-n1084861
3. “Juul Hit With Suit Over Teen’s Death From Respiratory Injury” https://news.bloomberglaw.com/product-liability-and-toxics-law/juul-hit-with-suit-over-teens-death-from-respiratory-injury?context=search&index=0&utm_medium=lawdesk&utm_source=twitter&campaign=8F28EC82-EF85-11E9-AF12-BDEC4F017A06
4. “SU student sues Juul after experiencing ‘catastrophic’ injuries” http://dailyorange.com/2019/09/su-student-sues-juul-experiencing-catastrophic-injuries/
5. “Ex-Juul executive alleges firm sent 1M contaminated pods to market” https://www.axios.com/juul-contaminated-pods-lawsuit-claim-c8c96421-cfd0-43e4-9e45-92fec1d0f6a8.html
6. “Vaping – The Next Wave of Lawsuits” https://www.natlawreview.com/article/vaping-next-wave-lawsuits
7. “‘It’s going to attack your lungs’: Gurnee teen hospitalized for vaping has message for his peers” https://www.chicagotribune.com/lifestyles/ct-life-teen-hospitalized-vaping-tt-20190904-73qpft3x5bc3zkbui7nrve4tsy-story.html
8. “Vapor Lung: Bronchiolitis Obliterans Organizing Pneumonia (BOOP) in Patient with E-Cigarette Use” https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A6513
9. “VpALIdVaping-related Acute Lung Injury: A New Killer Around the Block” https://www.mayoclinicproceedings.org/article/S0025-6196(19)30880-8/pdf
10. “Organizing pneumonia related to electronic cigarette use: A case report and review of literature” https://pubmed.ncbi.nlm.nih.gov/29392888/
11. “Possible Juul link to seizures kicks off FDA investigation” https://www.theverge.com/2019/8/29/20838907/juul-seizure-fda-risk-lung-disease-vape-e-cigarette
12. “Factbox: U.S. lawsuits take aim at vaping” https://www.reuters.com/article/us-health-vaping-lawsuit-factbox/factbox-u-s-lawsuits-take-aim-at-vaping-idUSKBN1ZT2CG
13. “The litigation environment in the vape industry” https://www.dlapiper.com/en/us/insights/publications/2020/04/the-litigation-environment-in-the-vape-industry/
14. “Bronchiolitis obliterans organizing pneumonia: Pathogenesis, clinical features, imaging and therapy review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2700454/
15. “Vaping Associated Lung Injury (EVALI): An Explosive United States Epidemic” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6913849/#:~:text=Vaping%20Associated%20Lung%20Injury%20(EVALI)%3A%20An%20Explosive%20United%20States%20Epidemic,-Gary%20A.
16. “Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI” https://www.nejm.org/doi/full/10.1056/NEJMoa1916433
17. “Juul Shipped At Least A Million Contaminated Pods, New Lawsuit Says” https://www.buzzfeednews.com/article/stephaniemlee/juul-lawsuit-contaminated-pods
18. “What We Know About Electronic Cigarettes” https://smokefree.gov/quit-smoking/ecigs-menthol-dip/ecigs#:~:text=E%2Dcigarettes%20are%20battery%2Dpowered,%2C%20flavorings%2C%20and%20other%20chemicals.
19. “NIH Drug Facts: Vaping Devices” https://www.drugabuse.gov/publications/drugfacts/vaping-devices-electronic-cigarettes
20. “How Electronic Cigarettes Work” https://science.howstuffworks.com/innovation/everyday-innovations/electronic-cigarette.htm
21. “The Three Main Reasons Youth Use E-Cigarettes” https://truthinitiative.org/research-resources/emerging-tobacco-products/3-main-reasons-youth-use-e-cigarettes
22. “FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint” https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children
23. “E-cigarette Ads and Youth” https://www.cdc.gov/vitalsigns/ecigarette-ads/index.html
24. “A closer look at vaping and e-cigarettes on college campuses” https://www.wildcat.arizona.edu/article/2019/11/n-vaping-health
25. “Did the FDA Ban E-Cig Flavors? Here’s What to Know” https://www.healthline.com/health-news/e-cig-flavor-ban-what-to-know
26. “Enforcement Priorities for Electronic Nicotine Delivery System (ENDS) and Other Deemed Products on the Market Without Premarket Authorization” https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-priorities-electronic-nicotine-delivery-system-ends-and-other-deemed-products-market
27. “Are Vaping and Juuling the Same Thing?” https://www.therecoveryvillage.com/teen-addiction/faq/are-vaping-and-juuling-the-same/
28. “Juul is under criminal investigation by federal prosecutors” https://www.theverge.com/2019/9/23/20880625/juul-criminal-investigation-ftc-fda-federal-probe
29. “E-Cigarettes: Current Evidence and Policy” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6140188/
30. “Xu X, Bishop EE, Kennedy, et al. Annual Healthcare Spending Attributable to Cigarette Smoking. American Journal of Preventive Medicine. 2015;48(3):326–333” https://www.sciencedirect.com/science/article/abs/pii/S0749379714006163
31. “Electronic Cigarettes: A Policy Statement From the American Heart Association. Circulation.” https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000107
32. “E-cigarettes: How “safe” are they? The Journal of Family Practice” https://www.mdedge.com/clinicianreviews/article/109243/addiction-medicine/e-cigarettes-how-safe-are-they
33. “Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation.” https://tobaccocontrol.bmj.com/content/23/suppl_3/iii3.short
34.“Progression of Poly-tobacco Product Use Patterns in Adolescents” https://www.sciencedirect.com/science/article/abs/pii/S0749379716300964
35. “U.S. Department of Health and Human Services . A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016. E-Cigarette Use Among Youth and Young Adults.” https://books.google.com/books?hl=en&lr=&id=Oy95lDRWJPUC&oi=fnd&pg=PA1&ots=tIjXD2x-EO&sig=U3wfXsGKmGqL1zBCWxTY-5oOOtQ#v=onepage&q&f=false
DePuy Synthes Orthopedics
One of the largest orthopedic and surgical device companies in the world, with a commanding market share of the total number of implanted hip and knee devices in the United States, DePuy Synthes Orthopedics is a business unit of global drug and device company, Johnson & Johnson. DePuy has one of the more comprehensive portfolios of orthopedic products and its primary focus is to provide options for reconstruction of damaged or diseased joints as well as other skeletal injuries. Despite its larger-than-life size and reputation, DePuy has also garnered its share of troubling headlines over the past 10-20 years concerning lawsuits stemming from its ASR and Pinnacle hip implant technologies. Indeed, hip implant technology verdicts and lawsuits numbering in excess of 20,000 parties have already cost the company more than $6 billion.
History and Background of DePuy Synthes
DePuy’s origins trace back to 1895 and the company’s founder, Revra DePuy, a chemist and pharmaceutical salesman from Warsaw, Indiana. Known then as the DePuy Manufacturing Company, it was established with the idea of manufacturing fiber splints that could be customized to fit patients and which were superior to the commonly fashioned wooden splints of the day.
DePuy continued to grow and following Revra DePuy’s death in 1921, the company shifted its focus to newer cutting-edge technologies fashioning artificial joints and spinal repair techniques. DePuy’s advances in these fields helped to shape the modern orthopedic device industry and transformed it into a preeminent leader in technology investment and innovation.
Johnson & Johnson acquired DePuy in 1998 for $3.5 billion and in 2012 coupled it with another acquisition of the firm Synthes. Last year alone, the DePuy Synthes unit reported $2.41 billion in revenue for Johnson & Johnson.
Issues with DePuy Hip Implant Technologies and Product Lines
DePuy ASR XL Acetabular System and ASR Hip Resurfacing Resurfacing Platform (MDL-2197)
DePuy manufactured the ASR Hip Implant Device line and is a business unit of the global device giant, Johnson & Johnson. The ASR XL Acetabular System was made up of three components:
- The metal femoral stem inserted inside of the femur
- The metal femoral head (or ball) connected to the stem; and
- The metal acetabular cup (socket)
Similarly, the ASR Hip Resurfacing Platform involves a metal cap placed over the natural femoral head and replacing the acetabulum with a metal cup.
Beginning in 2008, the FDA started receiving approximately 400 complaints from patients in the United States who were implanted with the devices. These patients complained of pain, swelling, inflammation, and damage to the bone and tissue – as well as a lack of mobility. Many of these patients required expensive and painful revision surgeries.
In 2010, DePuy issued a voluntary recall of ASR Hip Implant Devices and the first lawsuits naming DePuy soon followed thereafter. The cases were consolidated into an MDL and between 2013 and 2017, DePuy paid nearly $4.8 billion to settle approximately 7,500 cases.
It has been over ten years since the original ASR recall. And although this MDL is technically still open, settlement protocols have a stipulated deadline for potential litigants requiring revision surgery to have been performed no later than July 2017.
DePuy Pinnacle Hip Replacement System with TrueGlide Technology (MDL-2244)
The DePuy Pinnacle Acetabular Cup System was launched in 2001 and offered the option of either polyethylene or metal insert for use with a titanium cup to replace the natural hip socket. This was followed by the launch of the Pinnacle system coupled with TrueGlide technology in 2007.
Although the Pinnacle system was not subject to recall, patients still reported many of the same issues as those suffering from the DePuy ASR devices. Issues with loose implants, inflammation, swelling, and damage to surrounding tissue coupled with the spread of metal debris and contamination throughout the body were not infrequent. Many patients implanted with Pinnacle devices suffered the loss of mobility and went through painful and expensive revision surgeries in an attempt to correct issues.
Lawsuits involving the Pinnacle Hip Replacement System were aggregated into an MDL in a federal courtroom in Texas. In 2016, the second bellwether case in the MDL yielded a verdict in favor of the claimants to the tune of $502 million (later reduced under Texas law limiting punitive damages). A third bellwether case resulted in a $1 billion verdict in favor of claimants (reduced to $543 million on appeal) and a fourth bellwether case wound up with a $247 million verdict in favor of claimants.
In June 2019, DePuy announced that it would settle up to 6,000 of the Pinnacle lawsuits for $1 billion. DePuy is settling each case individually and as a consequence, this MDL remains open with up to 9,155 cases still pending.
Sources Cited (20)
1. “DePuy Synthes” https://www.jnjmedicaldevices.com/en-US/companies/depuy-synthes
2. “Our History” https://www.jnjmedicaldevices.com/en-US/companies/depuy-synthes/about-depuy-synthes/our-history
3. “DePuy Synthes Financial Statements” https://www.dnb.com/business-directory/company-profiles.depuy_synthes_inc.6dc63aa888fefa5e7a1271ab10b6d53f.html#financials-anchor
4. “$502 Million Dollar Verdict Against Johnson & Johnson In DePuy Pinnacle Hip Implant MDL” https://www.expertinstitute.com/resources/insights/502-million-dollar-verdict-against-johnson-johnson-in-depuy-pinnacle-hip-implant-mdl/#:~:text=The%20jury%20in%20this%20bellwether,%24360%20million%20in%20punitive%20damages.
5. “Lessons Emerging from Pinnacle Hip Bellwether Trials” https://www.lexology.com/library/detail.aspx?g=e8bb3be1-5777-48b8-a31f-3b9757ff2541
6. “In re: DePuy Orthopaedics, Inc. Pinnacle Hip Implant Products Liability Litigation” https://www.lexology.com/library/detail.aspx?g=e8bb3be1-5777-48b8-a31f-3b9757ff2541
7. “U.S. ASR Hip Settlement” https://www.usasrhipsettlement.com/
8. “Johnson & Johnson to Settle Hip Lawsuits for About $1 billion, Sources Say” https://www.latimes.com/business/la-fi-johnson-johnson-settle-pinnacle-metal-hip-implant-20190507-story.html
9. “Metal-on-metal: history, state of the art (2010)” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3032111/#:~:text=Typically%2C%20the%20first%20total%20hip,generation%20metal%2Don%2Dmetal.
10. “Hip Replacement Market to reach USD 10.51 Billion by 2026, Increasing Prevalence of Osteoarthritis to Boost Market, Predicts Fortune Business Insights” https://www.globenewswire.com/news-release/2019/12/09/1957817/0/en/Hip-Replacement-Market-to-reach-USD-10-51-Billion-by-2026-Increasing-Prevalence-of-Osteoarthritis-to-Boost-Market-Predicts-Fortune-Business-Insights.html
11. “DePuy Orthopaedics, Inc., ASR Hip Implant Products Liability Litigation” https://www.ohnd.uscourts.gov/mdl-2197
12. “Metal-on-Metal Hip Arthroplasty: A Review of Adverse Reactions and Patient Management” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4598667/#:~:text=Metal%2Don%2Dmetal%20relates%20to,metal%20acetabular%20cup%20or%20liner.
13. “NIH researchers uncover clues related to metal-on-metal hip implants” https://www.nih.gov/news-events/news-releases/nih-researchers-uncover-clues-related-metal-metal-hip-implants
14. “Metal-on-metal hip replacements: implications for general practice” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5697529/
15. “What is appropriate surveillance for metal-on-metal hip arthroplasty patients?” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5810829/
16. “Metal ion levels comparison: Metal-on-metal hip resurfacing vs total hip arthroplasty in patients requiring revision surgery” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6180337/
17. “Management of metal-on-metal hip implant patients: Who, when and how to revise?” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4865716/
18. “Heavy Metal? Recognizing Complications of Metal on Metal Hip Arthroplasty” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4158987/
19. “Management Guidelines for Metal-on-metal Hip Resurfacing Arthroplasty: A Strategy on Follow Up” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5525522/
20. “Outcomes of a metal-on-metal total hip replacement system” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4473440/
Ethicon Physiomesh
Physiomesh is a brand of synthetic, shaped, hernia mesh manufactured by Ethicon, a leading business unit/subsidiary of the global drug, medical device, and consumer products giant, Johnson & Johnson (J&J). Current J&J CEO, Alex Gorsky, actually came to the company from Ethicon following its acquisition. In 2019, J&J’s highly profitable surgical device division reported $9.5 billion in sales worldwide.
Ethicon marketed its flexible composite Physiomesh as a device to aid with laparoscopic hernia procedures. However, due to thousands of reports of adverse surgical outcomes sent to the U.S. Food and Drug Administration (FDA), Ethicon voluntarily withdrew Physiomesh for laparoscopic procedures in 2016. As of today, at least 2,820 hernia patients who underwent procedures with Physiomesh are now claimants in multidistrict litigation alleging they have suffered injuries and pain resulting from their being implanted with the device.
Background and History of Physiomesh
The use of surgical mesh to repair hernias dates back to the late 19th century and today is one of the most common surgical procedures performed in the United States. Among modern hernia surgeries, a mesh is used in more than 80% and there are more than 70 types of mesh products available on the market.
Physiomesh is made with a non-absorbent, macroporous polypropylene material that was aggressively marketed for both open hernia repairs as well as minimally invasive laparoscopic surgeries. Physiomesh has a unique design incorporating five (5) layers: two layers of poliglecaprone-25 (“Monocryl”) film covering two underlying layers of polydioxanone film (“PDS”), which in turn coat the polypropylene mesh. Like the vast majority of mesh products available in the United States, Physiomesh was fast-tracked for sale through the FDA’s 510(k) Clearance process.
What is Laparoscopic Hernia Repair Surgery?
There are three main types of hernia repair surgery available in the United States: “open”; laparoscopic and robotic. Open hernia repair is a more traditional method and involves making a sizable incision in the groin, where a surgeon pushes a hernia back, closes the abdominal wall with sutures, and then closes the incision with multiple sutures.
Conversely, laparoscopic hernia repair is a minimally invasive procedure that uses a thin, telescopic instrument (known as a laparoscope) inserted through the patient’s belly-button under general anesthesia. The abdomen is then inflated with gaseous carbon dioxide and the mesh is placed on the inside of the abdominal wall. Once completed, the incision is closed with one or two sutures or surgical tape. The ostensible benefit of laparoscopic surgery is a reduction in scarring and a shorter recovery time.

Flexible Physiomesh Complications
The market for synthetic mesh products is both profitable and competitive. Additionally, synthetic mesh devices, like Physiomesh, are almost always routed through the FDA”s fast-track 510(k) clearance process which does not require manufacturers to support marketing applications with large amounts of safety and efficacy data. Consequently, these products make it onto the market and into our bodies without much scrutiny and potential complications are sometimes overlooked.
Post-market surveillance of flexible (laparoscopic) Physiomesh indicated defects causing premature disintegration, tissue adhesion, contraction, and organ perforation. Due to the number of post-market “adverse event” reports coming back to Ethicon and the FDA, Ethicon voluntarily withdrew the flexible version of its Physiomesh line from the market in 2016. The “open” version is still available on the market for ventral hernia repair.
Physiomesh Lawsuits
Starting in 2017, patients claiming they were injured and suffering from implantation with flexible Physiomesh filed the first initial lawsuits naming Ethicon and J&J. As these lawsuits began to accumulate and grow in number, they were consolidated into multidistrict litigation in federal court in Georgia (MDL-2782). Today there are thousands of claimants integrated into the MDL and the first “bellwether” cases are expected to commence in late 2020. In addition to the MDL, several state cases are proceeding on another track in New Jersey.
Sources Cited (15)
1) “Ethicon Physiomesh for Open Ventral Hernia Repair” https://www.jnjmedicaldevices.com/en-US/product/open-ventral-hernia-repair-physiomesh-hernia-mesh
2) “Ethicon Hernia Portfolio: Evidence Summary” https://www.jnjmedicaldevices.com/sites/default/files/user_uploaded_assets/pdf_assets/2019-10/Ethicon-Hernia-Portfolio-Evidence-008101-180511.pdf
3) “J&J’s Ethicon recalls Physiomesh flexible composite hernia mesh” https://www.massdevice.com/jjs-ethicon-recalls-physiomesh-flexible-composite-hernia-mesh/
4) “Hernia Mesh Lawsuits: History and Developments” https://www.nolo.com/legal-encyclopedia/hernia-mesh-lawsuits-history-and-developments.html
5) “EARLY CLINICAL OUTCOMES OF HERNIA REPAIR WITH PHYSIOMESH” https://www.sages.org/meetings/annual-meeting/abstracts-archive/early-clinical-outcomes-of-hernia-repair-with-physiomesh/
6) “MAUDE Adverse Event Report: ETHICON INC, PHYSIOMESH” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=2543604
7) “MAUDE Adverse Event Report: ETHICON PHYSIOMESH” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/detail.cfm?mdrfoi__id=2363232&pc=FTL
8) “The Importance of Registries in the Postmarketing Surveillance of Surgical Meshes” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6250300/
9) “Nationwide Long-term Outcome Surveillance of Physiomesh® vs. Other Meshes in Laparoscopic Incisional Hernia Repair” https://clinicaltrials.gov/ct2/show/NCT03846661
10) “Class 2 Device Recall Surgical mesh” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=138394
11) “Hernia Repair Surgery” https://my.clevelandclinic.org/health/treatments/17967-hernia-repair-surgery
12) “Open surgery better than laparoscopic for repair of inguinal hernia” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC403835/
13) “LAPAROSCOPIC REMOVAL OF INGUINAL HERNIA MESH PLACED DURING A ROBOTIC TAPP HERNIA REPAIR IN A PATIENT WITH CHRONIC PAIN” https://www.sages.org/video/laparoscopic-removal-of-inguinal-hernia-mesh-placed-during-a-robotic-tapp-hernia-repair-in-a-patient-with-chronic-pain/
14) “Mind the gap: imaging spectrum of abdominal ventral hernia repair complications” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6439043/#:~:text=any%20abdominal%20surgery.-,Specific%20complications%20related%20to%20hernia%20repair%20include%20recurrent%20hernia%2C%20mesh,%2C%20fistula%20formation%2C%20and%20infertility.
15) “Johnson & Johnson: Annual Report 2019” https://www.investor.jnj.com/annual-meeting-materials/2019-annual-report
Davol (C.R. Bard) Polypropylene Mesh
Davol is a subsidiary of global medical device manufacturer, C.R. Bard, and is the primary brand manufacturer for C.R. Bard’s line of polypropylene surgical mesh devices. Founded in the 1870s as the Perkins Manufacturing Company, of Providence, Rhode Island, it was later renamed the Davol Rubber Company. Davol specialized in the production of rubber surgical devices and catheters and was acquired by C.R. Bard in 1980. Bard itself was acquired by medical device behemoth, Becton Dickinson in 2017 for $24 billion.
Together, Davol and C.R. Bard dominate up to 70% of the U.S. market for hernia mesh implants and have staked an aggressive marketing strategy on the use of polypropylene mesh for the range of products they have offered for many years. Nonetheless, polypropylene in the synthetic mesh has a well-documented history of post-operative complications. In particular, scientific evidence and studies have shown that polypropylene material is “biologically incompatible” with human tissue and may promote an immune response in some implantees resulting in contraction, adhesions, and migration around the abdomen. As a consequence, thousands of patients implanted with Davol/Bard polypropylene mesh products have come forward and are now suing the firms claiming that their products were defective, that the companies knew they were defective, and that they have caused devastating injuries and suffering.
Background on Davol/Bard and Polypropylene
The use of both organic and synthetic materials to repair hernias is a widely understood and adopted procedure in the United States and has been around since the late 19th century. In the United States alone, there are more than 300,000 mesh implant procedures conducted on an annual basis.
In recent years, device manufacturers have pursued a range of mesh products that incorporate a resin-based material known as polypropylene into their designs. The use of polypropylene in the implantable mesh is not without risks to the patient. Even though mesh manufacturers commonly assert that polypropylene is “inert”, the fundamental polymer in polypropylene undergoes chemical alteration following implantation in certain locations within the human body. Research into this alteration suggests that the synthetic material can degrade, crack, and shrink/contract causing infection, chronic inflammation, and other painful medical issues. These findings lead many to conclude that despite the manufacturer’s assurances, polypropylene is not “inert” when it is implanted in a human body.
C.R. Bard subsidiary, Davol, released its Composix Hernia Patch in 1997 which ushered in the firm’s contemporary line of the firm’s synthetic mesh devices made with layered polypropylene. Since that time, Davol’s list of synthetic mesh devices has grown to include (but not limited to):
- Composix Kugel Mesh
- Composix E/X Mesh
- Composix L/P Mesh
- Perfix Plug
- 3DMax
- Sepramesh IP Composite
- Ventralex Hernia Patch
- Ventralex ST Hernia Patch
- Ventrio Hernia Patch
- Ventrio ST Hernia Patch
- Visilex
- Marlex
- Spermatex
Issues and Complications from Polypropylene
Polypropylene mesh is commonly associated with chronic postoperative pain. It is believed that the mesh sometimes stiffens dramatically once it is implanted due to oxidative stress. Other issues and complications may include bleeding and infection, tissue adhesion, migration, bowel obstruction, and in some cases, erectile dysfunction.
Problems associated with the use of polypropylene in synthetic mesh devices are not new, either to the patients suffering from them or to manufacturers. According to evidence obtained during court proceedings in the United States, it emerged that Davol/C.R. Bard was told by the manufacturer of the resin they used that it was inappropriate for implantation in the human body.
Notably, on the Materials Safety Data Sheet (MSDS) for Marlex polypropylene published by the resin manufacturer Chevron Phillips Chemical Company, it states clearly: “Do not use this [manufacturer’s] material in medical applications involving permanent implantation in the human body or permanent contact with internal body fluids or tissues.” Beyond this admonition, a Vice President at C.R. Bard admitted in court that he received a call in 1997 from an executive at Chevron Phillips that, “…they (Chevron) were concerned about litigation and the association with the Marlex name with a permanent medical implant.”
Finally, in 2004, Chevron Phillips formally warned Davol/C.R. Bard and others that Marlex was “not for human implantation” and told Davol/C.R. Bard that they did not want to sell them Marlex at any price. Despite these warnings from Marlex’ own manufacturer, Davol/C.R. Bard continued using polypropylene in mesh devices for years afterward, allegedly going to great lengths to secure the resin while concealing its end purpose from suppliers.
Composix Kugel Patch Recall and Lawsuits
The Composix Kugel Patch was a mesh device marketed widely and aggressively by Davol/C.R. Bard in the 1990s through the mid-2000s. It was distinguished by a “memory recoil ring” that allowed the mesh to be inserted into the body through a small incision then unfold and lay flat. Unfortunately, the Kugel’s signature feature also demonstrated a propensity for breaking in the abdomen, causing bowel perforations and chronic intestinal fistulas. Between 2005 and 2007, the Kugel Patch was subject to a number of recalls, culminating with a Class I recall – the most serious type of recall mandated by the U.S. Food and Drug Administration (FDA). In the end, Davol/C.R. Bard had recalled nearly 140,000 units and paid $184 million to settle 2,600 lawsuits in 2017.
Current Davol/C.R. Bard Mesh Lawsuits
Davol/C.R. Bard are currently defendants in large multidistrict litigation (MDL) spanning a range of polypropylene devices in federal court in Ohio (MDL-2846). The first bellwether cases in this MDL are expected to open for trial late in 2020.
Sources Cited (14)
1) “MAUDE Adverse Event Report: DAVOL INC., SUB. C.R. BARD, INC. KUGEL PATCH” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/Detail.CFM?MDRFOI__ID=1749278
2) “Class 1 Device Recall Davol Composix Kugel Hernia Patch” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=49722
3) “Hansen v. Davol, Inc. and C.R. Bard” https://www.ohsd.uscourts.gov/multidistrict-litigation-2846
4) “Memos reveal mesh firms were warned 21 years ago that material should not be used on humans” https://www.sundaypost.com/fp/memos-reveal-mesh-firms-were-warned-21-years-ago-that-material-should-not-be-used-on-humans/
5) “Hernia Surgical Mesh Implants: Information for Patients” https://www.fda.gov/medical-devices/hernia-surgical-mesh-implants/hernia-surgical-mesh-implants-information-patients
6) “Gynecological mesh: The medical device that has 100,000 women suing” https://www.cbsnews.com/news/boston-scientific-gynecological-mesh-the-medical-device-that-has-100000-women-suing-2019-04-17/
7) “[Use of polypropylene mesh for incisional hernia repair]” https://pubmed.ncbi.nlm.nih.gov/16004221/
8) “Polyglactin/Polypropylene Mesh vs. Propylene Mesh: Is There a Need for Newer Prosthesis in Inguinal Hernia?” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3023108/
9) “Comparison of new biologic surgical mesh with polypropylene mesh in inguinal hernia repair” https://pubmed.ncbi.nlm.nih.gov/31142093/
10) “MAUDE Adverse Event Report: BARD DAVOL INC. BARD MESH MONOFILAMENT KNITTED POLYPROPYLENE, MESH SIZE 3″ X 6″” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/Detail.CFM?MDRFOI__ID=2048325&DEVICE_SEQUENCE_NO=2
11) “Physical structure and mechanical properties of knitted hernia mesh materials: A review” https://journals.sagepub.com/doi/abs/10.1177/1528083717690613?journalCode=jitc
12) “What is Polypropylene? The Plastic in Your Mesh” https://medtruth.com/articles/news/polypropylene-mesh/
13) “About Us: Davol/C.R. Bard” https://www.crbard.com/Davol/About-Us
14) “Post-Implantation Alterations of Polypropylene in the Human” https://www.researchgate.net/publication/224947996_Post-Implantation_Alterations_of_Polypropylene_in_the_Human#:~:text=Polypropylene%20in%20mesh%20form%20is,reactions%20after%20implantation%20in%20humans.&text=These%20alterations%20in%20the%20chemical,structural%20integrity%20through%20material%20embrittlement
Inferior Vena Cava (IVC) Filter Lawsuits and Updates
Last Updated September 28, 2020
An Inferior Vena Cava (IVC) filter is a small mechanical device placed into one of the largest vascular pathways in the human body to prevent clots from circulating from the lower limbs into the lungs causing a Pulmonary Embolism (PE). IVC filters are not necessarily “new” technology. They were first cleared for use by the U.S. Food and Drug Administration (FDA) through the abbreviated 510(k) Clearance process in the late 1970s. However, their use expanded dramatically in a very short span of time between 1999 and 2008.

In 2010, the FDA, after noticing a spike of “adverse event reports” through the previous years, issued a warning about IVC filters and the potential for such issues as filter migration; embolization; perforation of the Vena Cava; and mechanical fracturing of the filter itself. The FDA’s warning was followed in 2014 with a “safety communication” concerning the filters and the agency instituted a new postmarket surveillance study to evaluate the growing use of IVC filters and to make additional recommendations.
As of late 2020, nearly 15,000 lawsuits naming IVC manufacturers have been filed in multiple states, with the largest number of parties filing against manufacturers C.R. Bard and Cook Medical for their devices. These lawsuits have been consolidated into multidistrict litigation (MDL) in Arizona and Indiana.
Alleged Issues with IVC Filters
In recent lawsuits, patients implanted with IVC filters manufactured by Cook Medical and C.R. Bard alleged that due to their use of the 510(k) Clearance process, the companies failed to conduct any clinical testing or animal studies to determine how these filters would function once they are permanently implanted in the human body. Furthermore, the implantees alleged that Cook Medical and C.R. Bard knew or should have known that these devices are defective and dangerous; and that they have a high propensity for:
- Fracture
- Migration
- Excessive Tilting
- Perforation of the Vena Cava
Finally, the patients alleged that the companies were aware of defects with their filters in the face of increasing adverse reporting to the FDA. Nonetheless, they continued to aggressively market the devices while misrepresenting that they had much a better safety record than what was actually reported.
IVC Filter Manufacturers
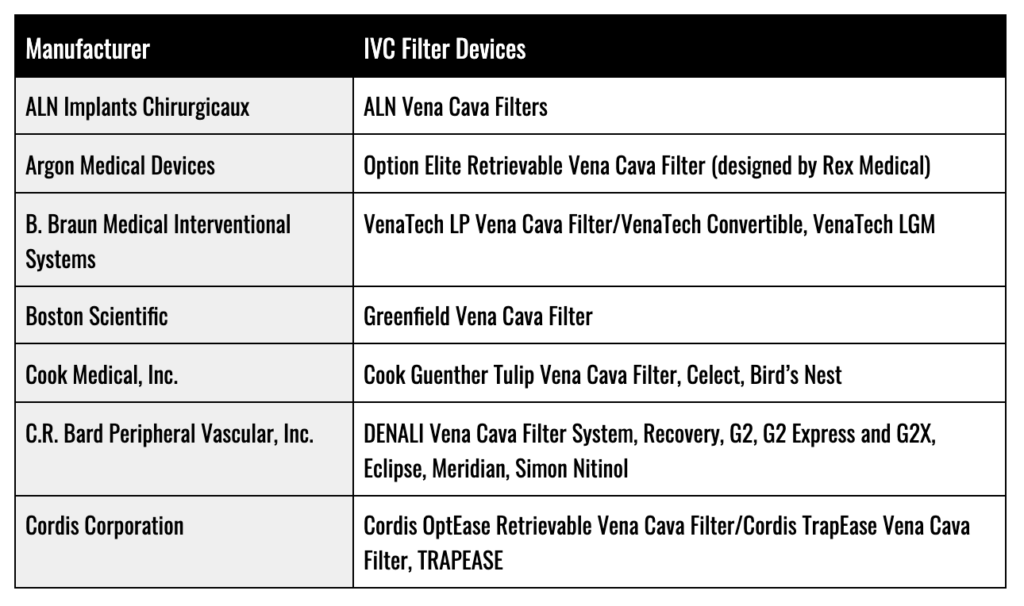
FDA Recalls and Administrative Action on IVC Filters
To date, the FDA has yet to issue any formal recalls for an IVC filter device. Nonetheless, between 2005 and 2019, at least eight types of IVF filters have been voluntarily withdrawn from the market by their manufacturers. In its first warning letter concerning IVC filters in 2010, the FDA detailed over 921 injuries from IVC filters spanning: migration through the body; migration into the heart and lungs (embolization); perforation of the vena cava; and filter fracturing. Since 2014, filter manufacturers have been given the option of either participating in the FDA’s 522 Postmarket Surveillance Studies program or an independent clinical study known as “Predicting the Safety and Effectiveness of Inferior Vena Cava Filters” (PRESERVE).
IVC Filter Lawsuits
In re: Bard IVC Filters (MDL-2641)
Lawsuits involving C.R. Bard’s Recovery and G2 IVC filter lines began emerging in late 2013 and early 2014. Due to the number and similarity of the allegations leveled in the complaints, they were consolidated into multidistrict litigation in Arizona. In their complaints, the implantees alleged that manufacturing and design defects in the Recovery and G2 lines, which they claimed C.R. Bard knew all along, were causing them to experience significant rates of fracture and migration. Some studies cited in the complaints stated that the Recovery line suffered from a 21% to 31.7% rate of fracture. Beyond the manufacturing and design defect claims, implantees also alleged that C.R. Bard actively denied and concealed knowledge of the dangers posed by the Recovery and G2 IVC filters and withheld such evidence in an attempt to delay court filings.
In March 2018, at the conclusion of the first bellwether trial for this MDL, a jury awarded plaintiff, Sherr-Una Booker $3.6 million in compensatory and punitive damages. Thereafter, C.R. Bard prevailed in two bellwether trials in June 2018 and October 2018. In May 2019, the judge presiding over this MDL announced a limited settlement for thousands of cases with the rest sent back to continue proceedings in state courts across the country. The MDL itself is now closed out, however, cases may still be brought in state court.
In re: Cook Medical, Inc. IVC Filters (MDL-2570)
Similar to the MDL involving C.R. Bard, cases revolving around the Gunther Tulip and Celect lines of IVC filters manufactured by Cook Medical, Inc., began to make their way into courtrooms around the country in 2013 and 2014. These cases were then consolidated into multidistrict litigation in Indiana. Like the plaintiffs in the C.R. Bard cases, the implantees in this MDL complained of alleged design and manufacturing defects such as tilt, migration, and fracturing. Specifically, some claimants referenced a study published in Cardiovascular Interventional Radiology in 2012 which assessed that the Gunther Tulip and Celect lines failed at a rate of 100% up to 71 days following implant and caused some degree of perforation of the wall of the vena cava. The same study reported that tilt was witnessed in 40% of the Gunther Tulip and Celect filter lines.
The outcomes of the various bellwether trials in this MDL have been a mixed-bag:
- In November 2017, a jury sided with Cook Medical and failed to find the company liable.
- A second bellwether case was dismissed in April 2018 due to the statute of limitation issues.
- A Houston firefighter was awarded $1.2 million by a Texas jury in May 2018 (not actually a bellwether case and not part of the MDL).
- In December 2018, the presiding judge in the MDL granted summary judgment to Cook Medical in a Georgia case brought by Tonya Brand.
- Most recently, in February 2019, an Indiana jury awarded $3 million to a woman who suffered a range of injuries from one of its IVC filter lines.
Sources Cited (20)
1. “Long-Term Clinical Outcomes of Complicated Retrievable Inferior Vena Cava Filter for Deep Venous Thrombosis Patients: Safety and Effectiveness” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6330022/
2. “Inferior Vena Cava Filter Placement and Removal” https://www.radiologyinfo.org/en/info.cfm?pg=venacavafilter
3. “Filter tilting and retrievability of the Celect and Denali inferior vena cava filters using propensity score-matching analysis” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6134324/
4. “About Your Inferior Vena Cava (IVC) Filter Placement” https://www.mskcc.org/cancer-care/patient-education/ivc-filter-placement
5. “What is the evidence behind the IVC filter?” https://emcrit.org/pulmcrit/what-is-the-evidence-behind-the-ivc-filter/#:~:text=37%20patients%20with%20recurrent%20venous,rate%20of%20IVC%20filter%20thrombosis.&text=Therefore%2C%20they%20concluded%20that%20this,two%20problems%20with%20this%20argument.
6. “Vena Cava Filters” https://my.clevelandclinic.org/health/treatments/17609-vena-cava-filters
7. “Vena cava filters: Tiny cages that trap blood clots” https://www.health.harvard.edu/heart-health/vena-cava-filters-tiny-cages-that-trap-blood-clots
8. “Inferior Vena Cava Filters: Guidelines, Best Practice, and Expanding Indications” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862857/#:~:text=There%20are%20two%20general%20types,and%20absolute%20contraindications%20to%20anticoagulation.
9. “Inferior Vena Cava Filter” https://www.ncbi.nlm.nih.gov/books/NBK549900/
10. “Permanent versus Retrievable Inferior Vena Cava Filters: Rethinking the “One-Filter-for-All” Approach to Mechanical Thromboembolic Prophylaxis” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862850/
11. “FDA Issues Statement on Treatment and Follow-Up Regarding IVC Filter Complications” https://evtoday.com/news/fda-issues-statement-on-treatment-and-follow-up-regarding-ivc-filter-complications
12. “Predicting the Safety and Effectiveness of Inferior Vena Cava Filters” http://www.preservetrial.com/
13. “Vena cava filters” https://www.medicalexpo.com/medical-manufacturer/vena-cava-filter-44592.html
14. “Complications of Inferior Vena Caval Filters” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3036364/
15. “IVC Filter Migration: How Does It Happen?” https://medium.com/@Drug_Justice/ivc-filter-migration-how-does-it-happen-1e453d77a0af
16. “FDA Updates Safety Communication on IVC Filter Retrieval” https://evtoday.com/news/fda-updates-safety-communication-on-ivc-filter-retrieval
17. “Lara L. Adams et al. v. Cook Medical Incorporated, et al” https://www.courtlistener.com/docket/5117387/adams-v-cook-medical/
18. “Charles Conn et al. v. C.R. Bard et al.” https://www.pacermonitor.com/public/case/9216987/Conn_et_al_v_C_R_Bard_Incorporated_et_al
19. “Perforation of the IVC: rule rather than exception after longer indwelling times for the Günther Tulip and Celect retrievable filters” https://pubmed.ncbi.nlm.nih.gov/21448771/
20. “Complications of Celect, Günther Tulip, and Greenfield Inferior Vena Cava Filters on CT Follow-up: A Single-Institution Experience” https://www.jvir.org/article/S1051-0443(13)01258-X/abstract
Democratic Lawmakers Blast Pharmaceutical Executives Over Drug Prices and Charitable Donations
During a House Oversight Committee hearing at the end of September, Democratic representatives harshly criticized pharmaceutical executives at Celgene, a Bristol Myers Squibb subsidiary, and Teva over drug prices and charitable contributions.
Rep. Katie Porter (D-CA) noted the disturbing similarities between bonuses that former Celgene CEO Mark Alles received in recent years and the price increases for Revlimid, a cancer therapeutic that cost 215 dollars in 2005 and 763 dollars today. Alles could not confirm that the higher price correlated with drug improvements, to which Porter responded, “To recap here: The drug didn’t get any better. The cancer patients didn’t get any better. You just got better at making money. You just refined your skills at price gouging.”
Rep. Rashida Tlaib (D-MI) considered internal documents from Teva that calculated the return on investment from charitable contributions and called out the drugmaker, saying, “Your pharmaceutical company makes these so-called charitable donations so you look like you give a shit about sick people.” Teva’s CEO Kare Schultz was so underprepared for the virtual hearing that one lawmaker told him he “might as well get off the screen,” while Rep. John Sarbanes (D-MD) said, “It would have been nice to come, maybe, equipped a little bit better.”
Further Reading:
Smith & Nephew to Acquire Integra’s Orthopedics Business
Smith & Nephew will acquire Integra LifeSciences’ orthopedics business in a 240 million dollar cash deal set to close at the end of 2020. The focus will be on growing the upper and lower extremity markets, adding products covering shoulder replacements and reconstructions of the hand, wrist, elbow, ankle, and foot. Through the acquisition, Smith & Nephew will also gain access to Integra’s next-generation shoulder replacement system, which is scheduled for a commercial launch in 2022. Additionally, it will bring a strong sales force and distributors to the United States, Canada, and Europe.
About 300 employees are expected to move from Integra, which is based out of Lyon, France, and Austin, Texas. Although sales decreased due to COVID-19 and the postponement of elective surgeries (down 48.6 percent in the second quarter of 2020 versus the same time in 2019), Smith & Nephew anticipates the business to deliver double-digit revenue growth in 2021 and 2022.
For Integra, the acquisition will allow the company to focus its efforts on developments in neurosurgery, surgical instrumentation, and regenerative medicine.
Further Reading:
Drug Law Journal's publishing and research are sponsored by the DDP Injury Law Group in Washington, D.C. Their legal team is focused on protecting the rights of injury victims.
Furthermore, they understand and appreciate the importance of a trusted attorney-client relationship.
The DDP Injury Law Group uses their years of experience with litigation to ensure their clients can fight for the compensation they deserve.
Always seek the advice of a medical professional when making personal health choices.
The Offices of DrugLawJournal.com are located at:
1800 North Orange Avenue, Suite C
Orlando, Florida 32804
DrugLawJournal.com is sponsored by the DDP Injury Law Group, and therefore may be considered attorney advertising. The information contained on DrugLawJournal.com is provided for informational purposes only, and should not be construed as legal or medical advice on any subject matter. No viewers of this site should discontinue taking a prescribed medication on the basis of any information on this site and should always first consult with a doctor concerning any medication. Viewers should understand that if they refrain from taking prescribed medication without appropriate medical advice they can suffer injury or death.
No viewers of content from this site, clients or otherwise, should act or refrain from acting on the basis of any content included in the site without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from an attorney licensed in the viewer’s state. Viewing information from DrugLawJournal.com does not create an attorney-client relationship between you and DDP Injury Law Group or DrugLawJournal.com nor is it intended to do so.The content of DrugLawJournal.com may not reflect current legal developments, verdicts or settlements. Prior results do not predict a similar outcome. For more information, please visit our web site’s disclaimer.
©2025 DrugLawJournal.com | Privacy Policy | Terms & ConditionsStay Informed
Sign up to receive peroidic updates from our expert team of researchers, highlighting defective drugs, devices, and legal issues related to your health.



