Last Updated November 3, 2020
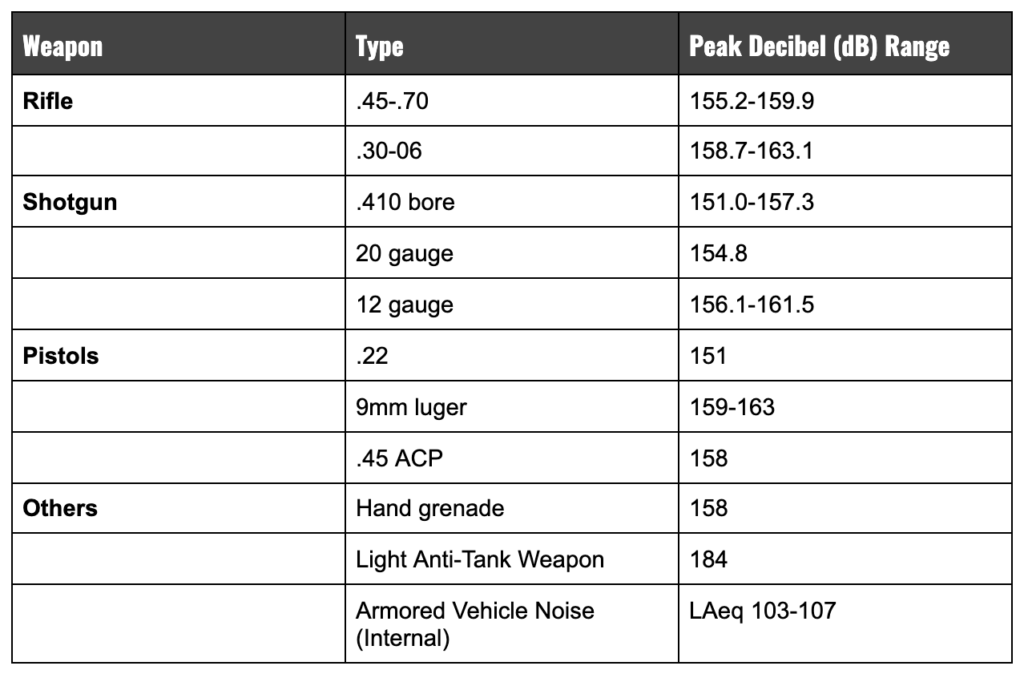
Hearing damage is a serious issue for U.S. Military veterans. More than 1.7 million veterans receive compensation for tinnitus and more than 1.1 million get it for hearing loss. According to the U.S. Department of Defense, combat veterans are sometimes exposed to sounds from rocket-propelled weapons, firearms, and explosions which can top 150 decibels. Consequently, veterans are 30% more likely than those who have never served to suffer severe hearing impairment.
Generally, hearing loss is considered preventable. If properly fit hearing protective devices are consistently and routinely worn under situations involving loud noise exposure, tinnitus and noise-induced hearing loss can be avoided. Otherwise, damage done to the hearing of veterans can be debilitating. Tinnitus can make it difficult to sleep, concentrate, or relax; and progressive hearing loss is linked to anxiety, depression, heart disease, and impaired cognitive function.
Hearing Loss Symptoms and Causes in Military Veterans
According to the Hearing Loss Association of America, there are three types of hearing loss:
Conductive Hearing Loss
Conducting hearing loss is usually characterized by a malformation in either the outer ear, ear canal, or middle ear structure. It can also be caused by other factors such as a severe ear infection, benign tumors, hereditary disorders, or even impacted ear wax. This type of hearing loss is generally treated with either surgery, antibiotic medications, or with the assistance of hearing devices, such as cochlear implants or hearing aids.
Sensorineural Hearing Loss
Sensorineural hearing loss is typically caused by factors such as exposure to loud noise, head trauma, viral infections/autoimmune responses, Meniere’s disease, otosclerosis, or just plain aging. Irreversible sensorineural hearing loss, the most common form, is usually treated with hearing aids or surgically implanted devices such as cochlear implants.
Mixed Hearing Loss
A combination of conductive damage in the outer or middle ear combined with sensorineural damage in the inner ear and/or the auditory nerve.
In addition to hearing loss, many veterans suffer and cope with Tinnitus. In fact, it is the number one disability among veterans and affects nearly 10% of American adults. Described as ringing/buzzing sounds, or high-pitched whistles – the origins or tinnitus can actually be attributed to a number of causes. It could result from close proximity to an Improvised Explosive Device (IED) blast, repeated exposure to long rifle fire engagements, long term exposure to loud equipment, or something as mundane as a switch in medication. However, it is known that tinnitus and hearing loss often go hand-in-hand and tinnitus is often the first symptom of hearing damage.
Veterans and those on active duty generally have a higher risk of permanent hearing loss. Common causes of hearing loss among veterans include:
Firearms and Explosive Weapons Noise
Constant exposure to the operation of some military weapons, especially those that create noise over 140 decibels will put anyone at greater risk for loss of hearing. Additionally, they can cause “high-frequency” hearing loss making it difficult to hear anything that is 2000 hertz or higher (high-pitched voices; noisy conversions in crowded rooms; use of consonants in speech).
Ship Engines and Carrier Deck Noise
Navy veterans, especially those involved with naval aviation, are at high risk due to the loud noise involved with engine room and carrier deck operations. Engine noise can frequently exceed 112 decibels and result in hearing loss later in life. On the other hand, jet engines routinely produce sounds in excess of 160 decibels creating greater risks for pilots and deck crews of high-frequency hearing loss and tinnitus.
Jet Fuel Exposure
Kerosene-based military-grade jet fuel (also sometimes referred to as JP-8) is made up of a combination of petrochemicals that when combusted, can produce fumes interfering with neurological pathways in the brain that decipher speech and language. Combined with other loud noise factors, jet fuel can increase a veteran or servicemember’s risk for later developing tinnitus or hearing loss.
Without a doubt, hearing protection devices are key to protecting the hearing of servicemembers against damaging noise levels which can result in hearing loss or tinnitus.
Typical hearing protection devices include:
- Triple/Quad Flange Earplugs
- Tactical Earplugs
- Tactical Communication and Protective Systems (TCAPS)
- Noise Muffs
- Foam Earplugs
According to the Hearing Center of Excellence, hearing protection devices do not ordinarily cause decreased situational awareness. Service members need to choose proper hearing protection based upon the environment, situation, noise type, and level of noise to which they are exposed.
3M Combat Arms Earplugs – Defective Protection Devices
Between 2003 and 2015, it is believed that the U.S. military purchased at least one pair of “Combat Arms” earplugs for every serviceman and servicewoman sent abroad for foreign deployment. Manufactured by a subsidiary of one of the world’s largest corporations, the 3M Company of Minnetonka, Minnesota, these earplugs were furnished to this nation’s fighting men and women for the ostensible purpose of protecting their hearing from the loud and often concussive sounds experienced during combat and training. However, veterans who were equipped with these devices now report that they experience a multitude of hearing issues which they believe stem from the defective design of the 3M Combat Arms earplugs.
In July 2018, 3M agreed to settle a whistleblower claim brought under the False Claims Act by a competitor in U.S. ex rel. Moldex-Metric, Inc. vs. 3M Co. The allegations in that suit focused on the false statements 3M and Aearon made to the U.S. Government concerning the defective nature of the earplugs. However, as part of the settlement, 3M made no admission as to its liability or wrongdoing.
The Veterans Administration and Hearing Loss
The Veterans Administration does provide benefits and compensation for service-related disabilities involving hearing loss and tinnitus. Typically veterans must establish that their hearing issues were caused by military duty and establish:
- A current diagnosis of a hearing condition
- Evidence of an event in service that caused the condition
- A medical opinion linking the hearing issue to the service-related event
The hearing condition diagnosis, in order to be deemed service-related, must be conducted by a licensed audiologist and involve two tests:
- Maryland CNC Test
- Puretone Audiometric Test
The Veterans Administration will use these auditory test results and its regulatory formula to determine an actual rating to assign. Typically, ratings for hearing loss are 0% or 10%. Severe or profound hearing loss can qualify for a higher rating.
Noise-Induced Hearing Loss Treatment
Noise-induced hearing trauma is usually permanent in nature and at the moment, cannot be remedied with surgery or procedures removing fluid or wax blockages. Standard options to treat or ameliorate noise-induced hearing damage to involve:
Hearing Aids
Damage to the inner ear and resulting hearing loss can be remedied with a hearing aid. An audiologist can review the service member’s case file and discuss options for a device type that will present the best fit.
Cochlear Implants
More severe types of hearing loss may be more suited to a cochlear implant. This device amplifies sound and directs it into the ear canal, bypassing damaged areas in the ear structure. Service members interested in this type of solution should consult their audiologist as well as an Ear-Nose and Throat (ENT) specialist.