Last Updated December 2, 2020
Generally, hip placement surgery – also known as Total Hip Arthroplasty (THA) enjoys a favorable rate of success in patients. However, even under the best of circumstances, THA can still fail for any number of reasons. If a hip replacement procedure is unsuccessful or does not otherwise meet expectations, a surgeon may recommend a follow-up procedure to either remove or replace some or all of the implanted device. This type of surgery is known as a “revision”
Reasons for Hip Revision Surgery
Patients who undergo THA can usually expect that their prosthesis will last at least 15-20 years – and in some cases, remain within the patient for the rest of their life. However, in approximately 18% of all hip replacement scenarios, doctors will recommend revision surgery. The most typical factors mitigating in favor of revision are:
Recurrent or Repetitive Dislocation of the Hip Joint
Traumatic hip dislocation can occur where the ball of the hip joint is pushed out of the socket. A dislocated hip can be debilitating if not addressed to put the ball and socket back into proper alignment and ensure proper complementation by surrounding muscle and ligament tissue.
Fortunately, dislocation is a relatively infrequent phenomenon among younger patients who follow proper care guidelines given by their physician. However, among elderly patients or those who have THA following fracture, rates of dislocation rise precipitously. Furthermore, patients who have already suffered dislocation are more likely to suffer additional dislocations without corrective surgery as surrounding tissue becomes increasingly disrupted.
Infection
Infection resulting from any surgical setting, including hip replacement, is a very real problem and a possibility all patients must face. Exposure to infectious bacterial agents can occur even in surgical theaters observing even the most stringent sterilization protocols. Additionally, patients who suffer from conditions such as diabetes mellitus, obesity, peripheral vascular disease, or are immunocompromised, may be at even greater than usual risk for infection.
The risk for infection is greatest within the first six weeks following surgery. If infection sets into the prosthesis, the surgeon will usually recommend a battery of tests to identify the type of infectious agent at work, possibly including an aspiration of joint fluid from the hip joint itself.
Once the surgical team identifies the type of infection, they will consider a range of treatment options. Chief among these is the use of targeted antibiotics and revision surgery to eradicate the infection. Typically revision procedures to address infection include:
- Surgical cleansing with intravenous antibiotics.
- A complete exchange of the hip replacement device:
- Two-Stage: The surgeon will remove the device and infection then clean the bone. A temporary cement spacer will be implanted while the patient undergoes a six-week course of intravenous antibiotics. Following the antibiotic treatment, a new prosthetic device will be implanted.
- Single Stage: This procedure entails the removal of the device, total cleansing of the infected joint, and replacement with a new device all at once. Like the two-stage procedure, patients will require six-to-eight weeks of intravenous antibiotics afterward.
Wear and Tear/Loosening
The metal, ceramic and plastic components of a hip joint replacement will rub against each other as part of the normal function of the prosthesis. Over time, however, joint movement can slowly wear down parts of the implant increasing the risk of failure or revision.
Typical factors contributing to excessive wear and/or loosening include:
- Excessive vigorous physical activity by younger/more active patients
- Obesity
- The type of device (i.e. “metal on metal” vs. “metal on polyethylene” vs “metal on ceramic”)
In some cases, wear and tear from the device will introduce synthetic particles into surrounding tissue as well as the patient’s bloodstream. These particles can then trigger an immune response that fosters progressively worse conditions such as osteolysis (destruction of surrounding bone tissue).
In Metal-on-Metal (MoM) hip implants, the metal ball and metal cup slide against each other during walking or running. This friction between the ball and socket can cause the release of metal particles which will wear off of the device and embed in surrounding tissue as well as metal ions of cobalt and chromium entering the bloodstream. Over time, this leaching of metal from the joint into surrounding tissue causes “Adverse Local Tissue Reaction” (ALTR) and/or “Adverse Reaction to Metal Debris” (ARMD). The bottom line for patients implanted with MoM joints, however, is that they suffer damage and pain, device failure, loosening, and the need for revision surgery. Furthermore, patients implanted with MoM hip joints can also suffer from a condition known as “metallosis” – a potentially fatal complication arising from metallic erosion in the joint which induces pain around the joint, pseudotumors, and a noticeable rash.
What to Expect with Hip Revision Surgery
Pre-Operative Protocols
In the weeks and days prior to revision surgery, doctors will order a variety of tests such as X-rays, CT and bone scans to identify the issues which they seek to address during the procedure. Additionally, they may ask for a routine aspiration of the joint to rule out infection risks prior to surgery. Patients should make arrangements for post-surgical assistance around the home and consult with their personal physician concerning medications or other relevant physical conditions. It is recommended that patients stop smoking as long as possible before surgery.
The Revision Procedure
A hip revision can be a longer and more complex procedure than the original THA surgery. A simple revision can just be a swap of liner components. A more complex revision can be as involved as a total replacement of all components. However, effective planning and coordination between the surgical team and your personal physician can help ward off more serious complications during and after the revision procedure.
Post-Operative Matters
Following revision surgery, patients may be allowed to sit up out of bed or even walk the very next day upon the advice of their surgeon. Some pain, but not excessive pain, is to be expected. Patients are typically discharged to their home or to a rehabilitation hospital within 5-7 days post-revision. Afterward, they will usually transition into a physical therapy regimen accompanied by the use of crutches and canes for walking assistance for as many as six weeks.
Complications and Risks from Revision Surgery
Revision surgery, like other major procedures, is not without inherent risk. Possible medical complications associated with any surgery include, but are not limited to:
- Allergic Reactions to Certain Medications
- Blood Loss
- Heart Attacks/Strokes/Kidney Failure/Pneumonia
- Nerve Damage
Risks more commonly associated with THA and revision surgery also include:
- Deep Vein Thrombosis (DVT) is a widely recognized risk factor following hip revision surgery. DVT is a blood clot in the leg that can break loose and become a Pulmonary Embolism (PE) that travels through the body and into the lungs where it can cause illness and death. DVT can occur in any vein in the body, however, they tend to manifest the most often in the lower extremities of the body.
- Infection following revision surgery is a possibility and a very serious problem. Accidental exposure to bacteria such as staphylococcus aureus can occur even in the midst of the most stringent sterilization protocols. Common postoperative infection symptoms include swelling, stiffness, pain, redness, fevers, night sweats, and chills.
Metal-on-Metal Hip Implants and Higher Rates of Revision Surgery
Starting in the early 2000s, several manufacturers aggressively marketed hip-joints through the 510(k) process featuring a “Metal-on-Metal” (MoM) coupling feature between the ball and socket of the joint. In MoM joints, the metal ball and metal cup slide against each other during walking or running. This friction between the ball and socket can cause the release of metal particles which will wear off of the device and embed in surrounding tissue as well as metal ions of cobalt and chromium entering the bloodstream.
The bottom line for patients implanted with MoM joints is that they suffer damage and pain, device failure, loosening, and the increased need for revision surgery. Furthermore, patients implanted with MoM hip joints can also suffer from a condition known as “metallosis” – a potentially fatal complication arising from metallic erosion in the joint which induces pain around the joint, pseudotumors, and a noticeable rash.
Recalls and Lawsuits of MoM Hip Replacement Devices
Smith & Nephew Metal-on-Metal and Hip Resurfacing
Smith & Nephew is a United Kingdom-based joint device manufacturer with operations in the United States. In 2017, Smith & Nephew was named a defendant in Multidistrict Litigation (MDL-2775) occurring in federal court in Maryland before Judge Catherine C. Blake. At the center of the litigation naming Smith & Nephew are several hundred claims alleging defects involving the company’s MoM Birmingham Hip Resurfacing (BHR) implants and its R3 hip joint products. The BHR was the subject of a recall for all units implanted between 2006 and 2015 due to high rates of failure and revision surgeries as was the R3 in 2012 due to issues with erosion and metallosis.
Stryker LFIT V40 Femoral Head and Rejuvenate/ABG II Hip Implant
Stryker is a Michigan-based medical device manufacturer that traces its founding back to Dr. Homer Stryker, an orthopedic surgeon, and his device inventions dating back to 1941. Today Stryker is an international giant that claims $14.9 billion in sales in 2019. In 2016, Stryker announced a recall of its LFIT V40 femoral heads manufactured before 2011 following post-market surveillance showing a higher-than-average rate of failure with symptoms in patients similar to those suffering from metallosis. Similarly, Stryker recalled its Rejuvenate and ABG II implants in 2012 (after only 2 years on the market due to post-market research indicating issues common to MoM devices. Both the LFIT V40 and the Rejuvenate/ABG II devices are the subject of ongoing multi-district litigation in federal court in Massachusetts (MDL-2768) and Minnesota (MDL-2441).
DePuy Orthopedics ASR Hip Implant and Pinnacle Hip Implant
DePuy Synthes is a subsidiary of global pharmaceutical and medical device manufacturer Johnson & Johnson. In 2010, DePuy Synthes issued a voluntary global recall for its ASR Acetabular System following evidence that emerged through post-market surveillance that patients were suffering considerable pain, immune system reactions, inflammation, and the need for revision. The symptoms were consistent with MoM injury and potential design flaws. Similarly, Depuy Synthes’ Pinnacle MoM hip implant is alleged to cause a condition called “osteolysis” which occurs when bone surrounding the hip joint dissolves due to instability and metallosis. Pinnacle has not been the subject of a recall as of this date. However, both the ASR Hip Implant and the Pinnacle Hip Implant are subjects of multi-district litigation in Ohio (MDL-2197) and Texas (MDL-2244).
Zimmer M/L Taper Hip Prosthesis and Versys Femoral Head (MDL-2859)
Indiana-based Zimmer Biomet Holdings, Inc. developed and marketed the Zimmer M/L Taper hip implant with its proprietary Kinectiv Technology. This model of the M/L Taper with the Kinectiv technology utilized a modular MoM design that featured a cobalt-chromium coupling. In court documents, claimants allege that the Zimmer hip joint suffers from corrosion which introduces metal fragments into surrounding tissue as well as elevated levels of metals into the bloodstream causing pain, swelling, tissue necrosis, and other ailments.
Sources Cited (20)
1) “Hip Revision” https://my.clevelandclinic.org/health/treatments/17104-hip-revision
2) “How Long Does It Take to Recover from Hip Replacement Surgery” https://www.thedacare.org/News-and-Events/Symptoms-and-Conditions/How-Long-Does-it-Take-to-Recover-from-Hip-Replacement-Surgery.aspx
3) “About Hip Revision Surgery” https://www.cedars-sinai.org/health-library/diseases-and-conditions/r/revision-hip-surgery.html
4) “Osteolysis” https://www.hss.edu/condition-list_osteolysis.asp
5) “Revision Total Hip Replacement: An Overview” https://www.hss.edu/conditions_revision-total-hip-replacement-overview.asp
6) “Revision surgery of metal-on-metal hip arthroplasties for adverse reactions to metal debris” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6055775/
7) “When to Have Hip Revision Surgery Due to Metal-on-Metal Prostheses” https://holycrossleonecenter.com/when-to-have-hip-revision-surgery-due-to-metal-on-metal-prostheses/
8) “REVISION HIP REPLACEMENT” https://www.stefankreuzermd.com/revision-hip-replacement.html
9) “Preventing Blood Clots After Hip or Knee Replacement Surgery or Surgery for a Broken Hip” https://www.ncbi.nlm.nih.gov/books/NBK107165/#:~:text=DVT%20is%20the%20most%20common,or%20weeks%20after%20the%20surgery.
10) “PREVENTION AND TREATMENT OF BLOOD CLOTS AFTER HIP AND KNEE REPLACEMENT SURGERY” https://www.stoptheclot.org/about-clots/toolkit-for-knee-hip-replacement-patients/prevention-and-treatment-of-blood-clots-after-hip-and-knee-replacement-surgery/
11) “Deep Vein Thrombosis” http://www.ucihealth.org/medical-services/orthopaedics/hip-knee-surgery-services/deep-vein-thrombosis
12) “BLOOD CLOTS IN ORTHOPEDIC SURGERY FACT SHEET” https://www.stoptheclot.org/about-clots/toolkit-for-knee-hip-replacement-patients/orthopedic-surgery-fact-sheet/
13) “Blood Clots After Surgery” https://www.webmd.com/dvt/blood-clots-after-surgery
14) “Hip Dislocation” https://www.hss.edu/condition-list_hip-dislocation.asp
15) “Dislocation Following Total Hip Replacement” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4298240/
16) “Risk factors for dislocation after primary total hip replacement: a systematic review and meta-analysis of 125 studies involving approximately five million hip replacements” https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(19)30045-1/fulltext
17) “Hip replacement” https://www.mayoclinic.org/tests-procedures/hip-replacement/about/pac-20385042
18) “Total Hip Replacement Fundamentals” https://my.clevelandclinic.org/health/treatments/17102-hip-replacement
19) “Signs & Symptoms” https://www.sahortho.com/joint/signs-symptoms#:~:text=Thigh%20or%20groin%20pain%20is,a%20sign%20of%20implant%20loosening.
20) “Hip Replacement Loosening Symptoms” https://www.verywellhealth.com/hip-replacement-loosening-2548623#:~:text=The%20most%20common%20cause%20of,This%20problem%20is%20called%20osteolysis.
Hernia Surgery & Mesh Treatment Options
Last Updated November 23, 2020
A hernia is a condition where an organ, intestine, or fatty tissue (typically in the abdomen) pushes through a weak spot in a surrounding muscle or connective tissue. They are usually caused by a combination of pressure and the development of the weak spot. Hernias are more common later in life – although they can manifest at birth. Factors contributing toward hernia development are obesity; lifting heavy objects; diarrhea or constipation; or persistent coughing/sneezing. Additionally, poor nutrition, smoking, and overexertion can contribute to the formation of a hernia.
The only way to repair a hernia is with surgery. Hernia surgery is one of the most common surgical procedures performed worldwide. The most straightforward method is for a surgeon to make an open incision into the abdomen and then attempt to repair the breach in the tissue wall with sutures. However, this type of procedure has a high rate of “recurrence” meaning that the sutures do not hold up well over time and further procedures become necessary.
As an alternative to sutures, surgeons prefer to use mesh devices as a tool to strengthen the area around hernias and make a repair that has less possibility of recurrence. Historically, surgical mesh has been used to repair hernias for well over a century in the United States. Today, more than 80% of hernia repair procedures performed in the U.S. involve mesh and there are more than 70 types of mesh commercially available.
Hernia Surgical Mesh Treatment Options
The most common type of hernia repairs involve inguinal hernias. Other types of repair procedures can involve the following types of hernias:
- Femoral
- Inciscional
- Ventral
- Umbilical
- Hiatal
For some types of hernias, surgeons may elect to take a “wait-and-see” approach if the patient is not experiencing any serious complications. Instead, they will opt, with the patient, to monitor the hernia to ensure it is not getting any larger or causing other issues.
When surgery is a necessity, doctors will opt for one of two incision protocols based upon the patient’s condition and needs. The choice of the incision will also inform their choice of mesh device.
- Laparoscopic: This involves the surgeon making a small incision in the abdomen which will allow for surgical tools to be inserted to effect hernia repair. Not all mesh devices are well-suited for this type of procedure.
- Open Repair Surgery: This is a more traditional incision near the hernia for the purpose of repairing weak muscle tissue. If sutures are used instead of mesh, the procedure is referred to as a “primary closure”
Among laparoscopic incisional procedures, surgeons will choose a repair “approach” to effectuate repair. The most common approaches involve the position of the hernia relative to the peritoneum – the tissue lining your abdominal wall. Accordingly, these approaches are referenced as:
- Transabdominal Preperitoneal (TAPP): The surgeon will go into the peritoneal cavity to place a mesh device over possible hernia sites.
- Totally Extraperitoneal (TEP): The surgeon will not enter the peritoneal cavity and mesh will be used to seal a hernia from outside of the peritoneum.
- Intraperitoneal Onlay Mesh Technique (IPOM): Introduced in the early 1990s, IPOM involves a small incision where gas is blown into the abdomen to get better visibility for the surgeon. Once surgical tools are inserted through the incision, surgeons can place and suture a hernia mesh directly onto the peritoneum where it can come into contact with the intestines or other abdominal organs.
Types of Hernia Mesh
Surgical hernia mesh is typically constructed either from animal tissue or entirely man-made (“synthetic”) materials. Synthetic mesh, such as those made from polypropylene, are made into “knitted” or “non-knitted” sheet formats. Furthermore, they can be “absorbable” or “non-absorbable” materials – or even a combination of the two. The animal-derived mesh is usually considered to be absorbable and will degrade over time while promoting new tissue growth to strengthen the hernia repair. Consequently, the absorbable mesh is not generally regarded as a long-term repair solution.

Potential Issues and Complications with Hernia Mesh
Most new mesh brands, in particular the synthetic ones, were “fast-tracked” for review by the U.S. Food and Drug Administration (FDA) through its abbreviated 510(k) clearance process which does not require stringent testing or clinical trials prior to implantation. In post-market surveillance, some common mesh brands have been associated with the following issues in patients:
- Migration: Movement of the hernia mesh within the body.
- Contraction: The mesh will collapse on itself and no longer cover the hernia location.
- Adhesion: Scar-like tissue creation that can cause the tissue to “stick” or bind together.
- Fistula: Leakage into body cavities or through the skin due to an abnormal connection between the organs or intestines.
- Perforation: A hole or the bowels or neighboring organs due to contact irritation or an abrasion from the mesh.
Some mesh devices have been the subject of recalls and litigation. If you were implanted with a hernia mesh product and you are now experiencing pain and/or having to undergo invasive revision or removal surgery, you may be interested to explore your options for receiving compensation for suffering and medical expenses.
Sources Cited (21)
1) “Everything You Want to Know About a Hernia” https://www.healthline.com/health/hernia
2) “Current options in inguinal hernia repair in adult patients” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3306028/
3) “Anatomy essentials for laparoscopic inguinal hernia repair” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5075841/#:~:text=The%20major%20procedures%20include%20intraperitoneal,are%20performed%20from%20different%20direction.
4) “Two different laparoscopic techniques for repairing a hernia in the groin” https://www.cochrane.org/CD004703/COLOCA_two-different-laparoscopic-techniques-for-repairing-a-hernia-in-the-groin
5) “Peritoneal Disorders” https://medlineplus.gov/peritonealdisorders.html#:~:text=Your%20peritoneum%20is%20the%20tissue,They%20include
6) “Past, Present and Future of Surgical Meshes: A Review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5618132/
7) “The clinical effectiveness and cost-effectiveness of open mesh repairs in adults presenting with a clinically diagnosed primary unilateral inguinal hernia who are operated in an elective setting: systematic review and economic evaluation.” https://www.ncbi.nlm.nih.gov/books/NBK326920/
8) “Hernia Surgical Mesh Implants” https://www.fda.gov/medical-devices/implants-and-prosthetics/hernia-surgical-mesh-implants
9) “Inguinal Hernia” https://www.mayoclinic.org/diseases-conditions/inguinal-hernia/symptoms-causes/syc-20351547
10) “Hernia” https://my.clevelandclinic.org/health/diseases/15757-hernia
11) “Stony Brook Medicine: FAQs about Mesh in Hernia Repairs — What Patients Need to Know” https://www.stonybrookmedicine.edu/hernia/resources/faqs-about-mesh-in-hernia-repairs
12) “Which mesh for hernia repair?” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3025220/
13) “Hernia Repair” https://www.health.harvard.edu/a_to_z/hernia-repair-a-to-z
14) “Mesh and No-Mesh Hernia Repair” https://www.medstarwashington.org/our-services/surgery/treatments/hernia/mesh-and-no-mesh-repair/
15) “The Many Types of Hernia Mesh Explained” https://michiganherniasurgery.com/posts/hernia-repair/the-many-types-of-hernia-mesh-explained/
16) “Chronic abdominal pain secondary to mesh erosion into cecum following incisional hernia repair: a case report and literature review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959323/
17) “Is surgical mesh safe for my hernia surgery?” https://www.ucihealth.org/blog/2018/06/mesh-hernia-repair
18) “Mind the gap: imaging spectrum of abdominal ventral hernia repair complications” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6439043/#:~:text=any%20abdominal%20surgery.-,Specific%20complications%20related%20to%20hernia%20repair%20include%20recurrent%20hernia%2C%20mesh,%2C%20fistula%20formation%2C%20and%20infertility.
19) “Hernia Surgical Mesh Implants: FDA Activities” https://www.fda.gov/medical-devices/hernia-surgical-mesh-implants/hernia-surgical-mesh-implants-fda-activities
20) “The regulatory ancestral network of surgical meshes” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6007828/
21) “Mesh materials and hernia repair” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5571666/
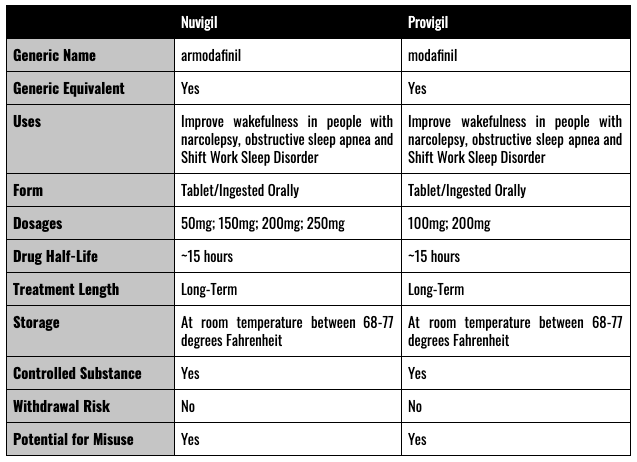
Provigil and Nuvigil Summary of Side Effects
Last Updated November 10, 2020
Provigil (modafinil) and Nuvigil (armodafinil) are both medications prescribed widely as treatments for extreme sleepiness or other sleeping disorders, which help patients stay awake during normal waking hours. It is believed that both drugs work by enhancing dopamine signals within the brain. However, the stark truth is that the actual wakefulness mechanism of either drug remains unknown. The more common side effects of Provigil and Nuvigil are documented and well established. However, recently, these drugs have been scrutinized further for potential associations with cardiac issues, birth defects, and Stevens-Johnson Syndrome. Both modafinil and armodafinil are listed as schedule IV controlled substances.
Documented Side Effects from Clinical Trials
Originally developed in the 1970s by a French professor in medical sciences, Michel Jouvet, modafinil began as an experimental treatment for narcolepsy in 1986. It wasn’t until 1998 that American drug manufacturer Cephalon gained approval from the U.S. Food and Drug Administration (FDA) to market Provigil in the United States.
According to modafinil’s FDA labeling, the drug was evaluated for safety in over 1,100 patients in placebo-controlled clinical trials and the most common adverse reactions (those affecting 5% or more) were: headache, nausea, dizziness, and insomnia. Beyond the significance of these findings, the FDA’s label also lists the following as some other side effects witnessed during clinical trials at a rate of 1% or more:
- Anxiety
- Diarrhea
- Dry Mouth
- Depression
- Dyspepsia
- Fatigue
- Palpitations
- Rash
- Upper Abdominal Pain
- Agitation
- Anorexia
- Constipation
- Contact Dermatitis
- Increased Heart Rate
- Migraines
- Thirst
- Tremors
- Vomiting
Subsequent Warnings and More Serious Side Effects
Birth Defects and Use by Women
Both Provigil and Nuvigil are classified as “Category C” drugs by the FDA – a designation that the agency, while concerned about their use during pregnancy, does not have enough evidence to suggest a clear safety hazard. Nonetheless, according to some reports, the rate of congenital malformations in babies connected with exposure to modafinil and armodafinil is as high as 15%.
Teva Pharmaceuticals Ireland and Health Canada issued pregnancy and birth defect warnings in 2019 covering Provigil and Nuvigil. The warnings urge healthcare providers not to prescribe either medication to women who are pregnant or who plan to become pregnant. In 2009, the FDA was concerned enough to establish a “pregnancy registry” for Provigil and Nuvigil to gather more information on the possible association of the drugs with fetal development. The registry tracks pregnant women exposed to at least one dose of either medication at least six weeks prior to conception and/or pregnancy.
Birth defects which have been associated with both Provigil and Nuvigil:
- Cleft Lip or Palate
- Hypospadias
- Congenital Heart Defects
- Microcephaly
Multi-Organ Hypersensitivity, Skin Rashes and Stevens-Johnson Syndrome
In 2007, after reviewing studies and adverse event reporting through its FAERS system, the FDA revised the labeling for both Provigil and Nuvigil to include information and warnings about serious rashes requiring hospitalization as well as Stevens-Johnson Syndrome (SJS).
SJS is a rare and severe skin reaction to a particular medication. It is an acute inflammatory condition linked with hypersensitive skin reactions. It is typically characterized by widespread blisters on the face and trunk of the body. These red/purple pustules which form over the body are intensely painful. As the reaction progresses, it takes up inside the mucus membranes and can spark blisters in the mouth, nose, eyes, and genitals. SJS requires hospitalization and the recovery time from an SJS reaction can persist from anywhere from several painful weeks to months.
In addition to SJS, the FDA’s label warning includes cautionary language concerning Toxic Epidermal Necrosis (TEN) – similar to SJS, and Drug Reaction with Eosinophilia (DRESS). DRESS, also referred to as “multi-organ hypersensitivity” is typically characterized by fever, rash, lymphadenopathy, and facial swelling alongside other maladies such as hepatitis, nephritis, and myocarditis.
Mental Health and Psychiatric Symptoms
During clinical trials for Nuvigil, some patients reported anxiety, agitation, nervousness, and irritability while on the drug. Other patients during trials reported issues with depression and suicidal ideation. The FDA expressed that caution should be exercised with Nuvigil when it is given to patients with a history of psychosis, depression, or mania and that if psychiatric symptoms emerge – patients and their physicians should consider discontinuing use. These same label warnings extend to Provigil as well.
Cardiovascular Health and Reactions
Some of the clinical subjects involved in the modafinil trial reported adverse cardiac reactions, including chest pain, palpitations, dyspnea, and transient ischemic T-wave changes on the electrocardiogram. The subjects involved also suffered from issues involving mitral valve prolapse or left ventricular hypertrophy. Accordingly, the FDA warns that patients suffering from these maladies should avoid Nuvigil and Provigil, especially if they have had prior issues with stimulants.
Analysis of studies conducted during trials of Nuvigil revealed that patients experienced “small average increases” in mean blood pressure. Another cohort of patients required new or increased use of blood-pressure medication to reduce hypertension. The FDA warns that caution should be exercised before prescribing either Nuvigil or Provigil to patients with known cardiovascular disease or similar issues.
Sources Cited (15)
2) “Armodafinil – FDA Labeling: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021875s023lbl.pdf
3) “Armodafinil – FDA Adverse Event Reporting System (FAERS)” https://drugcentral.org/drugcard/4501
4) “Armodafinil (Oral Route)” https://www.mayoclinic.org/drugs-supplements/armodafinil-oral-route/side-effects/drg-20071125?p=1
5) “Modafinil (Oral Route)” https://www.mayoclinic.org/drugs-supplements/modafinil-oral-route/side-effects/drg-20064870?p=1
6) “The Safety of Stimulant Medication Use in Cardiovascular and Arrhythmia Patients” https://www.acc.org/latest-in-cardiology/articles/2015/04/28/10/06/the-safety-of-stimulant-medication-use-in-cardiovascular-and-arrhythmia-patients#:~:text=Rare%20treatment%2Drelated%20cardiovascular%20events,40%2Dweeks%20of%20modafinil%20therapy.&text=Most%20common%20were%20palpitations%20(1.5,%2C%20and%20tachycardia%20(1.0%25).
7) “Modafinil-Induced Psychosis: A Case Report” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5353011/
8) “Modafinil Elicits Sympathomedullary Activation” https://www.ahajournals.org/doi/full/10.1161/01.hyp.0000158267.66763.63
9) “Preliminary Data Raises Concerns About Modafinil Use in Pregnancy” https://womensmentalhealth.org/posts/preliminary-data-raises-concerns-about-modafinil-use-in-pregnancy/
10) “ALERTEC (modafinil) and the Risk of Congenital Anomalies” https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2019/70201a-eng.php#:~:text=ALERTEC%20(modafinil%20100%20mg%20tablets,a%20circadian%20rhythm%20sleep%20disorder).
11) “Stevens-Johnson Syndrome After Armodafinil Use” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5940442/
12) “Stevens-Johnson syndrome” https://www.mayoclinic.org/diseases-conditions/stevens-johnson-syndrome/symptoms-causes/syc-20355936#:~:text=Stevens%2DJohnson%20syndrome%20(SJS),to%20heal%20after%20several%20days.
13) “Complications of Stevens-Johnson syndrome beyond the eye and skin.” https://eye.hms.harvard.edu/publications/complications-stevens-johnson-syndrome-beyond-eye-and-skin
14) “Conception, Pregnancy, Delivery, and Breastfeeding in a Narcoleptic Patient with Cataplexy” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2603540/
15) “FDA: Medication Guide – Provigil (modafinil)” https://www.fda.gov/media/79539/download
Provigil and Nuvigil Lawsuits and Updates
Last Updated October 27, 2020
Attorneys across the United States are taking notice of claims that popular “wakefulness” medications, Provigil and Nuvigil may be associated with increased incidences of birth defects. Although at the moment, no multidistrict litigation (MDL) or class-action lawsuits have been filed relative to these claims of birth defects, attorneys have taken it upon themselves to investigate these issues further and are accepting cases.

Provigil is the product name for the drug modafinil, an oral tablet that promotes wakefulness. Nuvigil is the product name for the drug armodafinil – also an oral tablet prescribed for patients having issues with daytime wakefulness. Provigil is manufactured by Teva Pharmaceuticals USA, Inc. and Nuvigil by Cephalon, Inc. (now a subsidiary of Teva). It is not known how either medication works to keep a person awake. However, they are thought to work by affecting certain substances in the brain that control the sleep and wake cycle through dopamine manipulation.
The FDA and Birth Defects
The U.S. Food and Drug Administration (FDA) classifies both Nuvigil and Provigil as “Category C” drugs. This means that the FDA has concerns about the use of both drugs during pregnancy, but will not state that a clear safety hazard exists on the basis of evidence. Nonetheless, both Nuvigil and Provigil have been associated with the following birth defects:
- Cleft Lip or Palate
- Hypospadias
- Congenital Heart Defects
- Microcephaly
In 2009, the FDA expressed enough concern about this association that it established a formal “pregnancy registry” for modafinil and armodafinil in order to gather additional information on the potential ramifications for pregnancy and fetal development.
Congenital Heart Defects
Congenital Heart Defects (also referred to as “CHD”) are present at birth in infants and can have a substantial impact on the function of how blood flows into a baby’s heart and through the rest of its body. Types of CHD include the following:
- Atrial Septal Defect
- Atrioventricular Septal Defect
- Coarctation of the Aorta
- Double-outlet Right Ventricle
- d-Transposition of the Great Arteries
- Ebstein Anomaly
- Hypoplastic Left Heart Syndrome
- Interrupted Aortic Arch
- Pulmonary Artesia
- Single Ventricle
- Tetralogy of Fallot
- Total Anomalous Pulmonary Venous Return
- Tricuspid Atresia
- Truncus Arteriosus
- Ventricular Septal Defect
Treatment options for CHD will depend upon the type and severity of the defect. Infants and children may require multiple surgeries to repair the heart or blood vessels. Furthermore, even though a heart defect can be repaired, treatment may not result in a cure. Children with CHD may develop other heart issues over time and require additional surgeries. With that in mind, however, many children born with CHD go on to lead independent lives with little or no difficulty.
Microcephaly
Microcephaly is a condition where a baby’s head is much smaller than is expected. This condition can occur because the baby’s brain has not developed properly during pregnancy or shortly after birth. In severe cases of microcephaly, a baby can be left with severe and life-threatening brain damage. Depending upon the severity, however, babies with microcephaly can also suffer from seizures; developmental delay; intellectual disability; problems with movement and balance; difficulty swallowing; hearing loss; and vision problems.
Microcephaly is a lifelong condition and there is no known cure or standard treatment. More severely affected babies will often require care and treatment focused on managing their health issues; as well as therapy to help maximize their physical and intellectual abilities.
Hypospadias
Hypospadias is a birth defect in boys impacting the position of the opening of the urethra. Babies born with hypospadias can form the opening to the urethra somewhere near the head of the penis (subcoronal); along the shaft of the penis (midshaft); or where the penis and the scrotum meet (penoscrotal). Additionally, hypospadias can result in curvature of the penis and abnormal spraying of urine. Hypospadias can be treated with surgery to correct the defect and is usually performed between the ages of 3-18 months old.
Cleft Lip and Cleft Palate
Children born with a cleft lip or cleft palate (sometimes in combination with each other) often have problems feeding and speaking clearly and may have regular ear infections. Both are birth defects that occur when a baby’s lip or mouth do not form properly during pregnancy. As a baby develops during pregnancy, facial tissue forms on each side of the head and join together to make the face. A cleft lip occurs when this tissue does not join completely before birth resulting in an opening in the upper lip. A cleft palate results when the tissue in the roof of the mouth similarly does not join together completely.
Depending upon the severity of the cleft and the existence of other birth defects, surgery can usually be an option for treatment within the first 12-18 months of life. With treatment, most children with clefts can expect to do well and live a happy life.
Anti-Trust Lawsuit
Although there are no large class-actions or MDLs involving birth defects yet, the manufacturers of Provigil and Nuvigil, Teva Pharmaceuticals, and Cephalon (along with generic manufacturers Mylan Laboratories and Ranbaxy Pharmaceuticals) recently paid over $134 million to settle an anti-trust lawsuit brought by the State of California. The lawsuit accused the companies of colluding to keep a generic version of Provigil from coming to the market sooner – a move which would have benefited consumers.
Sources Cited (16)
1. “The Nuvigil and Provigil Pregnancy Registry” https://clinicaltrials.gov/ct2/show/NCT01792583
2. “DailyMed: Nuvigil – Armodafinil” https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d878aed0-ddbf-8fa1-abf7-d3e480260845
3. “State of California v. Teva Pharmaceuticals, et al.” https://oag.ca.gov/sites/all/files/agweb/pdfs/antitrust/provigilsettlement/complaint.pdf
4. “Taking Provigil While Pregnant: What You Need to Know” https://www.therecoveryvillage.com/provigil-addiction/provigil-while-pregnant/
5. “Facts about Cleft Lip and Cleft Palate” https://www.cdc.gov/ncbddd/birthdefects/cleftlip.html
6. “Facts about Hypospadias” https://www.cdc.gov/ncbddd/birthdefects/hypospadias.html
7. “Facts about Microcephaly” https://www.cdc.gov/ncbddd/birthdefects/microcephaly.html
8. “The Safety of Stimulant Medication Use in Cardiovascular and Arrhythmia Patients” https://www.acc.org/latest-in-cardiology/articles/2015/04/28/10/06/the-safety-of-stimulant-medication-use-in-cardiovascular-and-arrhythmia-patients
9. “First-Trimester Pregnancy Exposure to Modafinil and Risk of Congenital Malformations” https://jamanetwork.com/journals/jama/fullarticle/2759460
10. “What are Congenital Heart Defects?” https://www.cdc.gov/ncbddd/heartdefects/facts.html
11. “Modafinil: Potential Risk of Congenital Malformations When Administered During Pregnancy” http://www.hpra.ie/docs/default-source/default-document-library/important-safety-information—modafinil99170c2697826eee9b55ff00008c97d0.pdf
12. “Practical Use and Risk of Modafinil, a Novel Waking Drug” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3286657/
13. “Health Canada Warns that Modafinil May Cause Fetal Harm” https://aasm.org/health-canada-warns-modafinil-fetal-harm/
14. “FDA Pregnancy Categories” https://chemm.nlm.nih.gov/pregnancycategories.htm
15. “Armodafinil While Pregnant: What You Need to Know” https://www.therecoveryvillage.com/armodafinil-addiction/armodafinil-while-pregnant/#gref
16. “Mechanisms of Modafinil: A Review of Current Research” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2654794/
Polypropylene Mesh Explained
Last Updated November 14, 2020
Hernia repair is one of the most common surgical procedures performed in the United States and it is estimated that over 20 million of them occur globally each year. Surgical meshes have been the preferred repair format by the medical profession for over 100 years and more than 80% of hernia repairs in the U.S. use mesh products. Accordingly, a great deal of research over the decades has been focused on developing new meshes made from both organic as well as synthetic materials.
Researchers and surgeons have examined the use of metals, polymers, and biodegradable materials for years as the basis for mesh that is simultaneously resistant to infection will maintain tensile strength, and will rapidly assimilate/incorporate into the surrounding tissue. In the 1950s, Dr. Francis Usher first examined the use of materials such as Nylon, Orlon, Dacron, and Teflon as possible mesh candidate materials. Usher eventually settled on a material known as Marlex and within two years introduced the first marlex-based woven hernia mesh.
On the basis of his work with Marlex, Dr. Usher later discovered that polypropylene mesh offered even more advantages due to its rapid incorporation into surrounding tissue and ease of sterilization. By 1998, research into polypropylene mesh materials had advanced enough that next-generation “lightweight” polypropylene hernia meshes were introduced into the market, largely through the U.S. Food and Drug Administration’s (FDA) “fast track” 510(k) clearance process.
Today there are more than 70 types of mesh on the market. However, despite their prevalence in hernia repair procedures, meshes – and in particular, ones made from polypropylene, have not all proven to be a huge benefit for patients. Some polypropylene mesh products have been linked with serious side effects that include severe pain, infections, tissue fusion, organ perforation, and worse. At the moment, three mesh manufacturers: Ethicon (Johnson & Johnson); Atrium Medical, and Davol (C.R. Bard) are embroiled in large-scale multidistrict litigation (MDL) concerning some of their best-selling hernia mesh products.
Polypropylene Basics
Polypropylene is a “thermoplastic” meaning that at high temperatures its form can be easily manipulated, extruded, or molded – later solidifying when it cools down. It is used in a variety of situations and consumer products ranging from essential parts used in the automotive industry to clothing and even in the surgical mesh.
Polypropylene is typically manufactured either through injection molding or using modern printing/cutting devices called “CNC” machines (Computer Numerical Control). It is preferred for its properties in the surgical setting which include: greater elasticity and toughness; as well as resistance to fatigue. Additionally, polypropylene is relatively inexpensive – making it even more desirable in the highly competitive and lucrative hernia mesh market.

Use of Polypropylene Mesh in Hernia Repair Procedures
Generally, surgical mesh is used to repair hernias in either a laparoscopic or open repair setting. In a laparoscopic procedure, a surgeon will make small incisions into the abdomen with surgical tools that can both repair the hernia and insert/set the mesh device. Open hernia repairs are more invasive and involve a larger incision in the abdomen to either suture the hernia or place a surgical mesh.
Polypropylene mesh is considered to be a “permanent” mesh material meaning it is non-absorbable. Permanent materials like polypropylene have virtues in their strength and ability to be easily manipulated. The size of the pores in polypropylene mesh can have a substantial impact on the reaction of surrounding tissue upon implantation. Smaller pores can result in a strong inflammatory response that can reduce the growth of surrounding tissue. Polypropylene hernia mesh made with larger pores can reduce the inflammatory response but inhibit fibrous tissue growth.
Polypropylene mesh can also be manufactured using a knitting or weaving process which will influence the size of pores and flexibility. Knitted mesh is more porous and flexible, while the woven mesh is generally stronger – but less adept at promoting fibrous tissue growth.
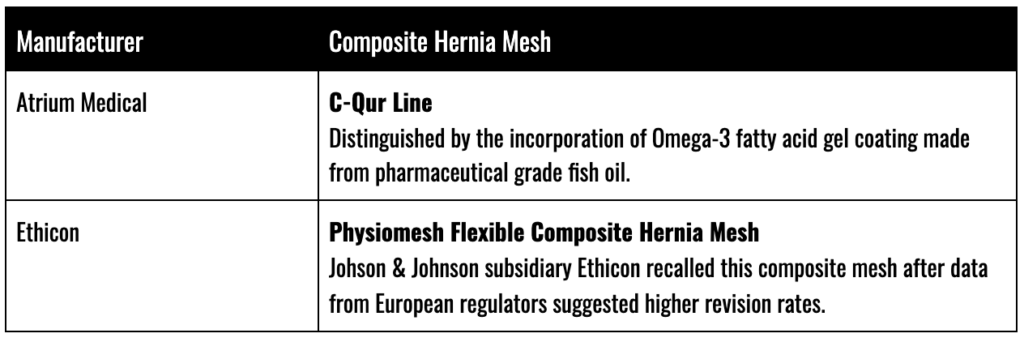
Permanent synthetic mesh materials like polypropylene are often combined with other absorbable materials to create “composite” meshes which, it is believed, will limit issues with inflammation, while promoting the strength and flexibility of the polypropylene. Typically, absorbable materials such as Dexon or Vicryl are used, which will completely degrade over time.
Examples of composite mesh products include:
Issues with Polypropylene in Hernia Repair
Most permanent meshes made with polypropylene were “fast-tracked” through the FDA’s 510(k) clearance process meaning that they went to market without rigorous testing or clinical trials. Many issues with synthetic permanent meshes were overlooked prior to implantation and many of them were not discovered until revealed in “post-market” surveillance required of manufacturers by the FDA. It is largely this type of ongoing review which has unmasked some of the more serious side effects and complications associated with polypropylene meshes.
Propylene Mesh Adhesion
Synthetic meshes made with polypropylene are associated with an increased risk of adhesion in patients. Adhesions are scar tissue that forms around the synthetic mesh and, in some instances, cause tissue in the abdomen to “stick” or bind together. This can result in small or large intestines clumping and pulling on each other causing intense chronic pain. Adhesion can also result in a serious abdominal infection known as peritonitis.
Adhesion can also cause either partial or complete bowel obstruction. Complete bowel obstruction can lead to infections such as sepsis and requires surgery to restore blood flow to the intestines.
Propylene Mesh Migration
Typically following laparoscopic procedures, polypropylene mesh can fail to properly adhere to surrounding tissue. This failure then leads to what is called “migration” as the mesh moves from its original insertion point and moves around the abdomen wreaking havoc on the abdominal cavity with infections and abscesses. In some cases, migration can only be remedied with additional surgery that can even be as dramatic as bowel resection.
Propylene Mesh Fistula
Fistulas are abnormal connections between abdominal organs and the intestines which can result when polypropylene mesh “erodes” into the gastrointestinal tract. Depending upon the location of a fistula, fluid can leak into body cavities and inhibit their normal performance. Fistulas can cause diarrhea or severe dehydration.
Propylene Mesh and Bowel Perforation
Bowel perforations are holes that develop in the large intestine which cause severe and even deadly abdominal infections when the contents spill out into the surrounding cavity. Surgeons will typically rush into surgery once a bowel perforation is detected and perform an ileostomy or colostomy to affect tissue healing.
Polypropylene Mesh Recalls and the FDA
Atrium C-Qur
The FDA warned Atrium, the manufacturer of the C-Qur mesh product line, of infection and sterility issues as far back as 2012. Most of the issues related to Atrium’s choice of the manufacturing production line and poor hygiene standards. After Atrium failed to remediate those issues, the FDA issued a Class II recall and sued the company over its poor quality control.
Ethicon/Johnson & Johnson Physiomesh Recall
Ethicon voluntarily recalled its Physiomesh products in 2016 on the heels of a spate of studies which suggested that the permanent synthetic meshes suffered from higher-than-normal rates of revision surgeries and complications.
Sources Cited (23)
1) “Past, Present and Future of Surgical Meshes: A Review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5618132/
2) “A History of Polypropylene Hernia Mesh and Its Many Problems” https://herniamesh.legalexaminer.com/technology/medical-devices-implants-technology/a-history-of-polypropylene-hernia-mesh-and-its-many-problems/
3) “Everything You Need To Know About Polypropylene (PP) Plastic” https://www.creativemechanisms.com/blog/all-about-polypropylene-pp-plastic
4) “Suture versus preperitoneal polypropylene mesh for elective umbilical hernia repairs” https://pubmed.ncbi.nlm.nih.gov/24980854/
5) “Current Issues and Challenges in Polypropylene Foaming: A Review” https://www.researchgate.net/publication/282672632_Current_Issues_and_Challenges_in_Polypropylene_Foaming_A_Review
6) “RECENT PROBLEMS AND FAILURES WITH PP MESH IMPLANTS HAVE LED TO THOUSANDS OF HERNIA AND SURGICAL MESH LAWSUITS” https://www.plasticexpert.com/plastic-technology/are-polypropylene-mesh-implants-safe/
7) “Surgical approach to abdominal wall defects: history and new trends” https://www.sciencedirect.com/science/article/pii/S1743919113600084
8) “Mesh infection in cases of polypropylene mesh hernioplasty” https://pubmed.ncbi.nlm.nih.gov/32096086/
9) “Complications of polypropylene mesh for the treatment of female pelvic floor disorders” https://pubmed.ncbi.nlm.nih.gov/21965260/
10) “Biologic versus Synthetic Mesh Reinforcement: What are the Pros and Cons?” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4477030/
11) “Polypropylene mesh implantation for hernia repair causes myeloid cell–driven persistent inflammation” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6413778/
12) “Mesh complications in female pelvic floor reconstructive surgery and their management: A systematic review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3424888/
13) “Urogynecologic Surgical Mesh Implants” https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants
14) “Polypropylene mesh implantation for hernia repair causes myeloid cell–driven persistent inflammation” https://insight.jci.org/articles/view/123862
15) “Recurrent urinary tract infection due to hernia mesh erosion into the bladder” https://www.researchgate.net/publication/7970758_Recurrent_urinary_tract_infection_due_to_hernia_mesh_erosion_into_the_bladder
16) “Surgical Mesh: Use and Complications in Women” https://my.clevelandclinic.org/health/articles/16298-surgical-mesh-use-and-complications-in-women
17) “Polypropylene mesh and systemic side effects in inguinal hernia repair: current evidence” https://pubmed.ncbi.nlm.nih.gov/30915679/
18) “Life after mesh: One patient’s harrowing experience with transvaginal mesh” https://www1.racgp.org.au/newsgp/clinical/life-after-mesh-one-patient-s-harrowing-experience
19) “Mesh Information for Patients – Frequently Asked Questions (FAQs)” https://centralcarolinasurgery.com/posts/news/mesh-information-for-patients-frequently-asked-questions-faqs/
20) “Mesh materials and hernia repair” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5571666/
21) “Late-onset Deep Mesh Infection: A Study of Eight Cases Detected from 2666 Consecutive Patients with Abdominal Wall Hernia Repairs” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4976579/
22) “The Many Types of Hernia Mesh Explained” https://michiganherniasurgery.com/posts/hernia-repair/the-many-types-of-hernia-mesh-explained/
23) “Hernia Surgical Mesh Implants: FDA Activities” https://www.fda.gov/medical-devices/hernia-surgical-mesh-implants/hernia-surgical-mesh-implants-fda-activities
E-Cigarette, Vaping, and Juul Bans
Last Updated November 16, 2020
E-Cigarettes and vaping surged onto the American market in 2007 and immediately enjoyed huge success with the public. Whatever success early e-cigarettes enjoyed, however, was nothing compared to the market gallop that followed the introduction of Juul and its particular brand of cartridge-based nicotine delivery. Juul’s rapid adoption by the vaping public accounts for about 40% of the e-cigarette market and in 2018 its parent company, Juul Labs, Inc. was valued at $15 billion.
Throughout vaping’s early days, e-cigarette manufacturers frequently touted their products as “safe” or “safer than tobacco”. These claims went largely unchallenged until recently. Now, e-cigarette users and their families publicly confront manufacturers about their products and the horrible suffering that they believe comes from vaping.
Additionally, public health officials throughout the United States have started to thoroughly document and study some of the associations they are seeing between vaping, addiction, and respiratory distress:
- The American Thoracic Society (ATS) published an article that studied the potential connection between e-cigarette use and Bronchiolitis Obliterans Organizing Pneumonia (BOOP) – a rare lung disorder that makes breathing more difficult.
- In November 2019, a respected medical journal documented its extensive look at the bleak long-term health picture for Vaping Associated Lung Injury (EVALI) in the United States. The Centers for Disease Control (CDC) and New England Journal of Medicine have both conducted research linking a thickening ingredient in some vaping products, Vitamin E Acetate, with EVALI. EVALI sufferers frequently complain of symptoms such as cough, chest pain, and shortness of breath.
- The December 2019 Mayo Clinic proceedings examined the potential for respiratory distress associated with vaping. Among its findings, the journal concluded that the incidence of vaping-related lung injury is increasing and that e-cigarette compounds present a litany of detrimental effects for human health.
With the rapidly advancing understanding of what vaping products are and the magnitude of the potential harm associated with them, federal and state authorities have stepped-up efforts to either limit access to vaping products or prohibit their use in most public settings.
Federal Regulation of E-Cigarettes and Juul
The U.S. Food and Drug Administration (FDA) has taken the lead role in federal efforts to regulate the e-cigarette and vaping industries. The agency designates vaping devices broadly as “Electronic Nicotine Delivery Systems” (ENDS). In September 2019, then-acting FDA Commissioner, Ned Sharpless, M.D., stated that after witnessing a steady decline in the number of children and young adults using tobacco products, the FDA was seeing a sharp increase in their adoption of ENDS use. Accordingly, oversight of ENDS by the FDA is now a top priority for the agency and involves: developing more science; educating the public, and aggressively enforcing rules on marketing and access to ENDS by children.
In particular, these are some of the ENDS strategies which form the FDA’s approach:
Restricting Youth Access to ENDS
As of August 8, 2016, it is illegal to sell ENDS to anyone under the age of 18 (in some states, the age ranges as high as 19 or 21). Retailers are also legally responsible to verify the age of anyone under the age of 27 purchasing ENDS.
FDA Review of ENDS Products
The Tobacco Control Act, which became effective in August 2016, requires that any ENDS product which wasn’t on the market as of February 15, 2007, must be authorized by the FDA in order to be on the market. Implementation of this requirement has been slow, and ENDS which were on the market before enactment of the Tobacco Control Act technically have more leeway with enforcement. However, in 2019, a federal court in Maryland ordered that applications for ENDS (as well as other tobacco products) were to have been filed with the FDA no later than May 12, 2020.
Preventing Youth ENDS Use
A key facet of the FDA’s ENDS strategy involves addressing the sharp increase in e-cigarette use by teenagers. Part of the agency’s “Youth Tobacco Prevention Plan” focuses on curbing access to tobacco product youth marketing. To date, the FDA has taken some serious action related to limiting youth access to ENDS:
- Working with eBay to remove Juul listings and prevent new ones.
- Requiring Juul and other ENDS manufacturers to submit documentation on marketing and research of ENDS.
- Issuing warning letters to companies which market e-liquids with misleading imagery and packaging that mimicked things appealing to children, such as candy; cookies; juice boxes, and kid-friendly cereals.
- Issuing warning letters to companies that manufacture/market/sell ENDS liquids with misleading labeling that imitates prescription cough syrup.
FDA Ban on Flavored Cartridges
On January 2, 2020, the FDA announced the ban of any flavored, cartridge-based ENDS product, other than tobacco or menthol flavorings. The agency has signaled that the ban will be an enforcement priority for the agency.
FDA Investigation Over Possible Juul LInk to Seizures
Bloomberg News obtained communications and memoranda between FDA officials in October 2018 detailing three cases of seizures alongside significant Juul use. While the FDA documentation did not detail any causality between Juul and the seizures, they believe that at a minimum there was an “association” with Juul. Over the next few months, the FDA uncovered an additional 32 reports of e-cigarette use that were believed to be linked to seizures. The FDA announced an investigation into the link between seizures and vaping in April 2019.
State and Local Government Bans on Juul and Other E-Cigarettes
In recent years, various state and local governments have taken a range of approaches to regulating Juul use and vaping in general. Most have adopted regulations limiting vaping in public places largely in the same fashion that they limit the use of tobacco publicly already.
Massachusetts became the first state to place restrictions on the sale of flavored tobacco, including menthol, in November 2019. It was followed in 2020 by New York, New Jersey, and Rhode Island. Later in 2020, California became the first state to ban the sale of flavored e-cigarettes and menthol cigarettes. New York, California, and New Jersey also ban vaping in public areas including bars and restaurants, similar to existing state tobacco use prohibitions. Additionally, these states have enacted statewide vaping bans that generally mimic preexisting bans on public smoking:
- Oregon
- Utah
- North Dakota
- Vermont
- Maine
- Delaware
Of the remaining states, many permit local municipalities to enact regulations limiting the sale of vaping implements and e-cigarette use. It is estimated that at least 300 local communities have taken up restrictions on vaping and e-cigarette use.
Sources Cited (42)
1) “Vaporizers, E-Cigarettes, and other Electronic Nicotine Delivery Systems (ENDS)” https://www.fda.gov/tobacco-products/products-ingredients-components/vaporizers-e-cigarettes-and-other-electronic-nicotine-delivery-systems-ends
2) “How FDA is Regulating E-Cigarettes” https://www.fda.gov/news-events/fda-voices/how-fda-regulating-e-cigarettes
3) “FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint” http://fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children
4) “STATES & LOCALITIES THAT HAVE RESTRICTED THE SALE OF
FLAVORED TOBACCO PRODUCTS” https://www.tobaccofreekids.org/assets/factsheets/0398.pdf
5) “Vaping Illness Update: FDA Warns Public to Stop Using Tetrahydrocannabinol (THC)-Containing Vaping Products and Any Vaping Products Obtained Off the Street” https://www.fda.gov/consumers/consumer-updates/vaping-illness-update-fda-warns-public-stop-using-tetrahydrocannabinol-thc-containing-vaping
6) “Juul hit with another state lawsuit for allegedly targeting kids” https://www.nbcnews.com/health/vaping/juul-hit-another-state-lawsuit-allegedly-targeting-kids-n1085901
7) “Public health groups fuming over Trump’s inaction on vaping flavor ban” https://www.nbcnews.com/health/vaping/public-health-groups-fuming-over-trump-s-inaction-vaping-flavor-n1084861
8) “‘It’s going to attack your lungs’: Gurnee teen hospitalized for vaping has message for his peers” https://www.chicagotribune.com/lifestyles/ct-life-teen-hospitalized-vaping-tt-20190904-73qpft3x5bc3zkbui7nrve4tsy-story.html
9) “Vapor Lung: Bronchiolitis Obliterans Organizing Pneumonia (BOOP) in Patient with E-Cigarette Use” https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A6513
10) “VpALIdVaping-related Acute Lung Injury: A New Killer Around the Block” https://www.mayoclinicproceedings.org/article/S0025-6196(19)30880-8/pdf
11) “Organizing pneumonia related to electronic cigarette use: A case report and review of literature” https://pubmed.ncbi.nlm.nih.gov/29392888/
12) “Possible Juul link to seizures kicks off FDA investigation” https://www.theverge.com/2019/8/29/20838907/juul-seizure-fda-risk-lung-disease-vape-e-cigarette
13) “What We Know About Electronic Cigarettes” https://smokefree.gov/quit-smoking/ecigs-menthol-dip/ecigs#:~:text=E%2Dcigarettes%20are%20battery%2Dpowered,%2C%20flavorings%2C%20and%20other%20chemicals.
14) “NIH Drug Facts: Vaping Devices” https://www.drugabuse.gov/publications/drugfacts/vaping-devices-electronic-cigarettes
15) “How Electronic Cigarettes Work” https://science.howstuffworks.com/innovation/everyday-innovations/electronic-cigarette.htm
16) “The Three Main Reasons Youth Use E-Cigarettes” https://truthinitiative.org/research-resources/emerging-tobacco-products/3-main-reasons-youth-use-e-cigarettes
17) “E-cigarette Ads and Youth” https://www.cdc.gov/vitalsigns/ecigarette-ads/index.html
18) “A closer look at vaping and e-cigarettes on college campuses” https://www.wildcat.arizona.edu/article/2019/11/n-vaping-health
19) “Did the FDA Ban E-Cig Flavors? Here’s What to Know” https://www.healthline.com/health-news/e-cig-flavor-ban-what-to-know
20) “Enforcement Priorities for Electronic Nicotine Delivery System (ENDS) and Other Deemed Products on the Market Without Premarket Authorization” https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-priorities-electronic-nicotine-delivery-system-ends-and-other-deemed-products-market
21) “Are Vaping and Juuling the Same Thing?” https://www.therecoveryvillage.com/teen-addiction/faq/are-vaping-and-juuling-the-same/
22) “Xu X, Bishop EE, Kennedy, et al. Annual Healthcare Spending Attributable to Cigarette Smoking. American Journal of Preventive Medicine. 2015;48(3):326–333” https://www.sciencedirect.com/science/article/abs/pii/S0749379714006163
23) “Electronic Cigarettes: A Policy Statement From the American Heart Association. Circulation.” https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000107
24) “E-cigarettes: How “safe” are they? The Journal of Family Practice” https://www.mdedge.com/clinicianreviews/article/109243/addiction-medicine/e-cigarettes-how-safe-are-they
25) “Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation.” https://tobaccocontrol.bmj.com/content/23/suppl_3/iii3.short
26) “Progression of Poly-tobacco Product Use Patterns in Adolescents” https://www.sciencedirect.com/science/article/abs/pii/S0749379716300964
27) “U.S. Department of Health and Human Services . A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016. E-Cigarette Use Among Youth and Young Adults.” https://books.google.com/books?hl=en&lr=&id=Oy95lDRWJPUC&oi=fnd&pg=PA1&ots=tIjXD2x-EO&sig=U3wfXsGKmGqL1zBCWxTY-5oOOtQ#v=onepage&q&f=false
28) “Banning Flavored JUUL Pods Is Actually Dangerous: Study Shows How Lawmakers Bungled Vape-Lung Crisis Response” https://www.forbes.com/sites/chrisroberts/2020/08/27/banning-flavored-juul-pods-is-actually-dangerous-study-shows-how-lawmakers-bungled-vape-lung-crisis-response/?sh=2904aecd430c
29) “JUUL Sales Recover Within Weeks of Self-Ban on Vaping Flavors” https://www.cancer.org/latest-news/juul-sales-recover-within-weeks-of-self-ban-on-vaping-flavors.html
30) “Teens Find a Big Loophole in the New Flavored Vaping Ban” https://www.nytimes.com/2020/01/31/health/vaping-flavors-disposable.html
31) “Flavored e-cigarette pod ban starts Thursday: What it means for vapers, kids and parents” https://www.washingtonpost.com/health/2020/02/05/flavored-e-cigarette-pod-ban-starts-thursday-what-it-means-vapers/
32) “Juul stops selling mint ahead of anticipated federal ban on most e-cigarette flavors” https://www.nbcnews.com/health/vaping/juul-stops-selling-mint-ahead-anticipated-federal-ban-most-e-n1077211
33) “The FDA bans most fruit- and mint-flavored nicotine vaping products to curb teen use” https://www.cnbc.com/2020/01/02/fda-issues-ban-on-some-flavored-vaping-products.html
34) “Juul Accepts Proposed Ban On Flavored Vaping Products As CEO Steps Down” https://www.npr.org/2019/09/25/764201798/juul-will-agree-to-ban-on-flavored-vaping-products-says-its-ceo-is-stepping-down
35) “FDA Bans Most Flavored Vape Pods. Altria’s Juul Stake Could Benefit, Analyst Says” https://www.barrons.com/articles/altria-juul-vape-pods-flavors-tobacco-menthol-fda-ban-51577977148
36) “Juul, e-cigarette maker, to drop ads, won’t lobby against proposed ban on flavored products” https://www.statnews.com/2019/09/25/juul-e-cigarette-maker-to-drop-ads-wont-lobby-against-proposed-ban-on-flavored-products/
37) “Mass. Just Lifted Its Ban On Vaping Product Sales. Here’s What You Need To Know” https://www.wbur.org/commonhealth/2019/12/10/juul-vapes-ban-massachusetts
38) “Juul Labs Donate to Politicians Despite Threats of E-Cig Bans” https://www.vapingpost.com/2020/07/01/juul-labs-donate-to-politicians-despite-threats-of-e-cig-bans/
39) “FDA BANS SALE OF PUFF BAR E-CIGARETTES, THE ‘NEW JUUL’” https://www.truthinadvertising.org/fda-bans-sale-of-puff-bar-e-cigarettes-the-new-juul/
40) “House approves bill to ban the sale of flavored e-cigarettes” https://abcnews.go.com/Health/wireStory/house-approves-bill-ban-sale-flavored-cigarettes-69286647
41) “JUUL Tricks Its Customers Into Advocating for the Florida Flavor Ban” https://vaping360.com/vape-news/89303/juul-tricks-its-customers-into-advocating-for-the-florida-flavor-ban/
42) “Juul will stop selling flavoured vapes in Canada. Here’s why that matters” https://www.cbc.ca/kidsnews/post/juul-will-no-longer-sell-flavoured-vaping-pods-in-canada-heres-why
Valsartan FDA Recall
Updated December 17, 2020
Valsartan is a medication that is part of a broader class of drugs known as Angiotensin II Receptor Blockers (“ARBs”). Originally, Valsartan was marketed in the United States under the brand name Diovan, although it is now widely available in its generic format. It is usually prescribed as part of a regimen to combat high blood pressure and hypertensive disorders. However, it can also be used to combat issues associated with Chronic Kidney Disease (CKD) and End-Stage Renal Disease (ESRD) in patients suffering from type-2 diabetes.
ARBs like Valsartan are very popular and have been prescribed to millions of Americans over the years. The medical community considers Valsartan to be a valuable tool to help combat a range of cardiovascular and other maladies frequently associated with type-2 diabetes.
Very recently, however, Valsartan’s generally unblemished reputation has come under intense scrutiny after certain batches of generic medication were found to be contaminated with N-Nitrosodimethylamine (NDMA), an extremely potent carcinogen. It is believed that improper manufacturing processes at plants in Zhejiang, China, and Telangana, India, are to blame for the NDMA contamination. These factories were outsourced by generic drug manufacturers to maximize the profitability of their brands and operated with little or no oversight or monitoring for safety and quality. Consequently, contaminated medications made their way through the supply chain and into batches circulated through the United States, Canada, and Europe.
As a result, in 2018, the U.S. Food and Drug Administration (FDA) began a series of voluntary recalls and alerted consumers and the medical community to be alert about contamination that may have already made it onto the market.
Drugs and Manufacturers Subject to the FDA Recall
It is very important to remember that not every batch of Valsartan was contaminated with NDMA. On certain batches and certain manufacturers were subject to testing and recall. Some of the manufacturers included on the FDA recall list include:
- Teva Pharmaceuticals (Major Pharmaceuticals on the label)
- Prinston Pharmaceutical, Inc. (Solco Healthcare, LLC on the label)
- Mylan Pharmaceuticals, Inc.
- RemedyRepack, Inc.
- Teva Pharmaceuticals USA/Actavis
- Torrent Pharmaceuticals Limited
- AvKare (Teva/Actavis)
- A-S Medication Solutions, LLC
- Bryant Ranch Prepack, Inc.
- NuCare Pharmaceuticals, Inc.
- H.J. Harkins Company, Inc.
- PharmaPac
- Hetero Labs, Inc.
- Preferred Pharmaceuticals, Inc.
- Northwind Pharmaceuticals, Inc.
- Aurobindo Pharma USA, Inc.
The complete list of recalled batches and manufacturers can be found here.
What is the Cancer Risk from NDMA Contamination?
NDMA is an organic chemical that is listed by the International Agency for Research on Cancer (IARC) as a group 2A carcinogen, meaning it is likely to cause cancer in humans because research has shown it to cause cancer in animals. In animal studies, NDMA has been shown to be acutely toxic to rats and even short bursts of exposure can lead to multiple organ damage in other animals. NDMA can occur both in nature or as the result of industrial manufacturing processes.
With that in mind, the FDA has offered some perspective on the risk associated with contaminated ARBs. In its updated announcement, the agency estimated that if 8,000 patients took 320mg of valsartan (the highest dose) contaminated with NDMA, there may be one additional cancer case during the lifetimes of those patients. The estimate is based upon average impurity levels in a single tablet of valsartan.
Additionally, the risk for developing certain types of cancer in humans can be attributed to a number of factors: genetic predisposition; environment; smoking, drinking, and eating habits; and obesity. It is, therefore, difficult to pin the risk for a specific type of cancer on NDMA contamination in Valsartan.
How Can I Tell if I Have a Recalled Batch of Valsartan?
Consumers can search the FDA’s Valsartan recall website here to find out which lot numbers were impacted by the recall as well as read the FDA’s official announcement. Next, they will want to look at their own drug containers to see if there is information indicating that they are part of a recalled lot.
Bottles and Vials:
Drug bottles will usually have a lot number printed next to the expiration date, either next to the barcode or underneath the dosing instructions.
Blister Packs:
Medication in blister packs will usually have the lot numbers and expiration dates printed on the foil side of the backings.
Cream and Gel Tubes:
Expiration dates and lot numbers on medications sealed in tubes, such as creams and gels, will usually be positioned at the end furthest from the cap – near what is called the “crimp”.
What Should I Do if I Have Recalled Valsartan?
If you believe you have contaminated or recalled Valsartan, you should immediately contact your physician. Make sure you have your packaging and prescription information ready for them in case he or she asks for it.
Your physician will be able to give you guidance on what next steps to take to replace your medication as well as discuss any symptoms you may be experiencing. Furthermore, your physician can give you advice on how to properly dispose of any contaminated medication.
Sources Cited (13):
1) “Search List of Recalled Angiotensin II Receptor Blockers (ARBs) including Valsartan, Losartan and Irbesartan” https://www.fda.gov/drugs/drug-safety-and-availability/search-list-recalled-angiotensin-ii-receptor-blockers-arbs-including-valsartan-losartan-and
2) “FDA Updates and Press Announcements on Angiotensin II Receptor Blocker (ARB) Recalls (Valsartan, Losartan, and Irbesartan)” https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan#:~:text=Teva%20is%20recalling%20all%20lots,N%2DNitrosodiethylamine%20(NDEA).
3) Use of N-nitrosodimethylamine (NDMA) contaminated valsartan products and risk of cancer: Danish nationwide cohort study” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6134800/#:~:text=Valsartan%20is%20an%20angiotensin%20II,treat%20hypertension%20and%20heart%20failure.&text=In%20July%202018%2C%20some%20valsartan,N%2Dnitrosodimethylamine%20(NDMA).
4) “FDA Expands Valsartan Products Recall” https://www.pharmacytimes.com/resource-centers/cardiovascular-health/fda-expands-valsartan-products-recall
5) “Valsartan, Losartan & Other BP Med Recalls 2018-19” https://www.webmd.com/hypertension-high-blood-pressure/valsartan-losatran-bp-med-recalls-2018-19
6) “NDMA, a contaminant found in multiple drugs, has industry seeking sources and solutions” https://cen.acs.org/pharmaceuticals/pharmaceutical-chemicals/NDMA-contaminant-found-multiple-drugs/98/i15
7) “What We Know about the Possible Carcinogen Found in Zantac” https://www.scientificamerican.com/article/what-we-know-about-the-possible-carcinogen-found-in-zantac/
8) “Does NDMA Cause Cancer?” https://www.verywellhealth.com/ndma-cancer-risk-5083965
9) “N-Nitrosodimethylamine” https://pubchem.ncbi.nlm.nih.gov/compound/N-Nitrosodimethylamine
10) “Use of N-nitrosodimethylamine (NDMA) contaminated valsartan products and risk of cancer: Danish nationwide cohort study” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6134800/
11) “A survey of feeding N-nitrosodimethylamine (NDMA) to domestic animals over an 18 year period” https://pubmed.ncbi.nlm.nih.gov/7228298/
12) “Dietary intakes of nitrate, nitrite and NDMA in the Finnish Mobile Clinic Health Examination Survey” https://pubmed.ncbi.nlm.nih.gov/8799716/
13) “Inflammatory bowel disease stimulates the formation of carcinogenic N-nitroso compounds” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1774505/
Vaping Cessation Assistance
Last Updated November 7, 2020
Adult use of combustible tobacco products, such as cigarettes has been on a continuous decline in the United States since the first Surgeon General’s report was released in 1964. In 2018, cigarette smoking among adults in the U.S. fell to 14%, the lowest on record for available data. However, this trend has been augmented by e-cigarettes, sometimes referred to as: e-cigs; vapes; e-hookas; vape pens; or by the brand name “Juul”; which entered the market in the U.S. around 2007. As of 2018, about 3.2% of American adults reported that they are regular e-cigarette users. Approximately half of adults who use e-cigarettes also smoke combustible tobacco – a behavior known as “dual-use”.
For many years, the use of e-cigarettes and vaping was touted (largely by manufacturers) as a safe alternative to smoking, and a useful tool to help with tobacco smoking cessation. While much remains to be learned about the long term health consequences of vaping, evolving research suggests that the increased exposure to nicotine, in combination with the aerosolized compounds released into the lungs by vaping, nonetheless present the potential for serious health consequences.
Because vaping is a much more sophisticated and efficient method for the delivery of nicotine, quitting can be quite a challenge for adults as well as teens. Nicotine addiction is a well-documented phenomenon as is nicotine withdrawal. Accordingly, if you are thinking about quitting your vaping habit – it helps to understand the effects of nicotine as well as have a plan for successfully countering nicotine withdrawal.
How Addictive Is Vaping?
E-cigarettes contain nicotine just like regular cigarettes. Research on nicotine suggests it may be as addictive as heroin and cocaine. Because of the vaporizing mechanism in e-cigarettes, users tend to receive much higher concentrations of nicotine than they would from an ordinary tobacco cigarette. Furthermore, users of e-cigarettes can even buy extra-strength nicotine cartridges or augment the voltage of the e-cigarette to increase the amount of nicotine.
Nicotine is dangerous for adults as well as teens. However, it presents particular problems for early brain development when it is used by teens. Adolescent brains tend to be more sensitive to “rewards” which trigger dopamine in younger brains. Dopamine is a well-understood neurotransmitter that gives the brain a “feel-good” feeling and encourages the brain to repeat actions that promote dopamine release. The more dopamine triggers in the brain, the more association with nicotine rewards become engrained. When addicted e-cigarette users, and in particular, teens, attempt to quit vaping, nicotine cravings become a huge problem in the absence of a consistent dopamine rush.
What Are the Potential Dangers?
Compared to regular cigarettes and the volumes of research that have emerged over several decades, e-cigarettes have only been on the market for a relatively short amount of time. Even so, doctors and scientists have been successfully accumulating copious research on e-cigarettes in an effort to understand more fully what their impact is on human health.
Nicotine Exposure
Even short-term exposure to nicotine has been demonstrated to include tremors and an increase in heart rate, blood pressure, and respiration. Nicotine is a teratogen meaning it can promote the growth of tumors. Furthermore, nicotine exposure for pregnant mothers and small children can have long term impacts on fetal brain development or even potentially lethal consequences if ingested.
BOOP
The American Thoracic Society (ATS) published an article on the prevalence of Bronchiolitis Obliterans Organizing Pneumonia (BOOP) in patients claiming they use e-cigarettes. BOOP is a rare inflammatory lung disorder involving flu-like symptoms and coughing that make breathing very difficult in sufferers.
EVALI
The Journal of the Missouri State Medical Association’s November 2019 edition gave an extensive look into the increasingly bleak outlook for Vaping Associated Lung Injury (EVALI) in the United States. The articles noted that as of its publication, there were 2,051 cases of e-cigarette or vaping lung product uses associated with lung injury (with 39 reported deaths) in the U.S. Overwhelmingly, EVALI patients complain of symptoms like cough, chest pain, and shortness of breath. Of the documented cases expressing EVALI, 86% of the patients reported using THC-containing products. In February 2019, the New England Journal of Medicine and the Centers for Disease Control (CDC) had both established research pieces linking Vitamin E Acetate, a thickening fluid used predominantly in THC vaping products with EVALI.
How Difficult Is It to Quit Vaping?
Again, due to the short amount of time most e-cigarette products have been on the market, hard data on vaping and quitting is mostly still emerging. However, the American Lung Association has noted that it is observing people having a greater difficulty trying to quit vaping than they did with combustible cigarettes. E-cigarette users tend to inhale more deeply and hold the vapor for longer periods of time than ordinary tobacco smoke. Accordingly, they tend to dose the brain with substantially more nicotine, more frequently throughout the day. Consequently, the symptoms and effects of nicotine withdrawal on e-cigarette users may even be more profound than they are on ordinary tobacco smokers.
While the U.S. Food and Drug Administration (FDA) has approved nicotine patches, gum and lozenges to help quit combustible tobacco use, no similar method has been approved to assist vapers with quitting.
What Are Some Methods for Quitting Vaping?
Because nicotine is addictive, the human brain and body become conditioned to its presence. For most users of nicotine, removal of nicotine from their bodily systems will come with some immediate side effects. For e-cigarette users serious about quitting, it helps to first reconcile all of the reasons why you should quit – and then have a plan for how you are going to address and cope with some of the side-effects of kicking the nicotine habit.
Step One – Tell Yourself Why You Want to Quit
Determining why you want to quit using nicotine is usually a good place to start on the path to successfully kick the habit. Perhaps you are concerned with the litany of potential ill-health effects connected with vaping. Maybe you’re thinking that if you quit, you’ll save a lot of money or help protect loved ones from second-hand exposure to nicotine. There is no wrong reason to quit.
Step Two – Understand the Challenges That Come with Nicotine Withdrawal
In the absence of nicotine after vaping, the body and mind must adjust. This adjustment is called nicotine withdrawal and it is usually accompanied by nicotine cravings. Typical nicotine withdrawal symptoms can include:
- Feeling irritable
- Headaches
- Increased sweating
- Anxiety
- Sadness
- Feeling tired
- Difficulty with concentration
- Insomnia or difficulty sleeping
- Increased hunger
- Intense cravings for e-cigarettes
Step Three – Have a Plan to Cope with Nicotine Withdrawal
Withdrawal symptoms will vary from person-to-person. Some will enjoy success going “cold-turkey” while others will struggle with cravings and other symptoms. With that in mind, cravings will tend to wear off over time the longer your body has to adjust to being nicotine-free.
Some tips for creating a strategy to kick the habit include:
- Build a Support Team
You don’t have to deal with nicotine withdrawal alone. Reach out to family and friends to let them know what’s happening and what type of support you would appreciate from them. Remember to always be gracious for their help.
- Talk to Your Physician
Ask your doctor if he or she has any ideas or support resources to help you kick the vaping habit.
- Exercise
Physical activity is an excellent way to stave off craving impulses. Jogging, lifting weights, or going for short walks can help release brain chemicals which will give you the same “feel good” impulse as the dopamine cycle triggered by nicotine.
- Coping with Stress
Vaping is frequently a solution for users who feel “stressed out”. This leads to a vicious cycle of cravings. Cultivate meditation or deep breathing exercises to help you better cope with stressful situations to avoid the temptation to vape.
- Distractions
Cravings are usually a moment-to-moment problem, and generally, only last a minute or two. Finding distractions, like reading, listening to music, or solving a puzzle can help divert your mind long enough for the craving symptoms to pass.
- Acknowledge Your Success
Take time to recognize your success getting out from under the yoke of vaping. Whether it’s the first few hours or the first 1,000 days – be sure to congratulate yourself.
Sources Cited (16)
1) “How to Quit Vaping” https://teen.smokefree.gov/quit-vaping/how-to-quit-vaping
2) “Ready to Ditch Vaping? 9 Tips for Success” https://www.healthline.com/health/how-to-quit-vaping
3) “Quitting vaping? Here are 5 tips for handling nicotine withdrawal” https://truthinitiative.org/research-resources/quitting-smoking-vaping/quitting-vaping-here-are-5-tips-handling-nicotine
4) “6 Steps to Quit Vaping or Smoking” https://www.psychologytoday.com/us/blog/working-through-shame/201906/6-steps-quit-vaping-or-smoking
5) “Vaping Addiction and Nicotine Withdrawal” https://teen.smokefree.gov/quit-vaping/vaping-addiction-nicotine-withdrawal#:~:text=Nicotine%20is%20in%20most%20vapes,help%20manage%20your%20withdrawal%20symptoms.
6) “5 Vaping Facts You Need to Know” https://www.hopkinsmedicine.org/health/wellness-and-prevention/5-truths-you-need-to-know-about-vaping
7) “Adult Smoking Cessation—The Use of E-Cigarettes” https://www.cdc.gov/tobacco/data_statistics/sgr/2020-smoking-cessation/fact-sheets/adult-smoking-cessation-e-cigarettes-use/index.html
8) “Electronic Cigarettes” https://www.cdc.gov/tobacco/basic_information/e-cigarettes/index.htm
9) “Quit Smoking” https://www.cdc.gov/tobacco/quit_smoking/index.htm
10) “Vaping Illness Update: FDA Warns Public to Stop Using Tetrahydrocannabinol (THC)-Containing Vaping Products and Any Vaping Products Obtained Off the Street” https://www.fda.gov/consumers/consumer-updates/vaping-illness-update-fda-warns-public-stop-using-tetrahydrocannabinol-thc-containing-vaping
11) “Want to Quit Smoking? FDA-Approved Products Can Help” https://www.fda.gov/consumers/consumer-updates/want-quit-smoking-fda-approved-products-can-help
12) “ELIMINATING YOUTH ELECTRONIC CIGARETTE AND OTHER TOBACCO PRODUCT USE: THE ROLE FOR DRUG THERAPIES” https://www.fda.gov/media/120342/download
13) “E-Cigarettes are More Addictive than Traditional Cigarettes—A Study in Highly Educated Young People” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6651627/#:~:text=The%20nicotine%20dependence%20levels%20measured,1.6%3B%20p%20%3C%200.001).
14) “Nicotine Addiction From Vaping Is a Bigger Problem Than Teens Realize” https://www.yalemedicine.org/stories/vaping-nicotine-addiction/
15) “Four E-cigarette Health Risks for Kids” https://www.nm.org/healthbeat/healthy-tips/emotional-health/vaping-4-risks-for-kids
16) “How Hard Is It to Quit Vaping?” https://elemental.medium.com/how-hard-is-it-to-quit-vaping-c58cf754d913
Proton Pump Inhibitors Summary of Side Effects
Last Updated November 9, 2020
Proton Pump Inhibitors (PPIs) are drugs first developed in the mid-1970s which are designed to limit the production of digestive acid by blocking a key enzyme in the stomach wall. Specifically, PPIs stop certain cells in the stomach lining from pumping or producing acid. Since first gaining approval by the U.S. Food and Drug Administration (FDA), PPIs such as omeprazole and esomeprazole have experienced extreme popularity both in their prescription and over-the-counter (OTC) formats with patients. Prilosec (omeprazole) generated over $26 billion for AstraZeneca before falling “off-patent” – only to be replaced with Prilosec (esomeprazole) which generated another $48 billion in sales for the company.
Without a doubt, PPIs have been a huge commercial success and millions have used them as part of a comprehensive strategy to combat peptic ulcer disease, non-ulcer dyspepsia, and Gastroesophageal Reflux Disease (GERD) among other digestive afflictions. However, PPIs also come with a range of possible side effects: some common, others not so common. Furthermore, recent studies regarding the long-term use of PPIs have suggested possible adverse side-effects which have the potential to be life-altering or worse.
More Common Side Effects
Documented side effects for PPIs include the following:
- Headache
- Nausea
- Stomach Pain
- Gas
- Constipation
- Diarrhea
- Rash
- Vomiting
- Constipation
- Fever
- Cold or Flu-like Symptoms
Other Possible Serious Side Effects
Acute Interstitial Nephritis, Chronic Kidney Disease, and End-Stage Renal Disease
Recent reporting and studies have raised concerns about a possible association between PPI use and acute injury to the kidneys. In particular, several case reports have suggested a link between PPIs and the development of Acute Interstitial Nephritis (AIN). AIN is a kidney disorder in which the spaces between the kidney tubules become inflamed and swollen. Symptoms of AIN associated with PPI use include:
- Malaise
- Fever
- Nausea
- Lethargy
- Weight Loss
If left unchecked, AIN can progress to Chronic Kidney Disease (CKD), a condition that encompasses the gradual loss of kidney function. Unfortunately, the symptoms of CKD are not always readily apparent for sufferers and may not become obvious until kidney function has already been substantially impaired. While the onset of CKD is gradual, its effects are serious and it brings a substantially increased risk of death from cardiovascular events.
Long-term suffering with CKD can regrettably lead toward End-Stage Renal Disease (ESRD) otherwise known as complete renal failure. ESRD occurs when a person’s kidneys are no longer able to function properly. ESRD treatment options require either frequent dialysis or a kidney transplant for the patient to survive.
Note: in 2014 the FDA required prescription PPIs to carry warnings on their labels concerning the risk of AIN.
Hypomagnesemia
Hypomagnesemia, otherwise known as magnesium deficiency, is a condition where this important element’s presence in the blood is lower than normal. Magnesium is critical to the operation of the heart, lungs, and kidneys among other critical organs, and is of central function to the body’s metabolism. In 2011, the FDA issued a safety warning concerning the association between PPI use and magnesium deficiency and recommended monitoring of magnesium levels in patients who have been on PPIs for a long duration.
Clostridium Difficile Associated Diarrhea
In 2012, the FDA issued a safety communication concerning increased risk from PPIs and Clostridium Difficile Associated Diarrhea (CDAD). Clostridium difficile is a bacteria that can cause serious diarrhea that does not improve. After reviewing reports from the FDA’s Adverse Event Reporting System (AERS), the agency noted that many reports of patients undergoing treatment with PPIs and who manifested CDAD suggested the possibility of a linkage. It should be noted that most of the reporting involved elderly patients who were also taking doses of broad-spectrum antibiotics or had other conditions that may predispose them to CDAD. However, the FDA could not rule out the role of PPIs in the development of CDAD.
Small Intestine Bacterial Overgrowth
Small Intestine Bacterial Overgrowth (SIBO) is a condition where there is an abnormal increase in the bacterial population of the small intestine. SIBO can occur as a result of surgery or disease and can cause diarrhea, weight loss, and malnutrition. The Mayo Clinic recently reported that PPI use may also carry an increased-risk for SIBO in patients.
Cancer Risk from Long Term Use
There are no definitive research or medical links between PPI use and cancer. However, two studies conducted in 2017 and 2018 included data and conclusions that support further research into any potential relationship between PPIs and gastric cancer. Specifically, researchers at the University of Hong Kong and others in Sweden conducted similar studies examining the relationship between PPIs and H. Pylori infections in the gastrointestinal system. H. Pylori are bacteria that live in the digestive tract and cause ulcers and increase the risk of stomach or esophageal cancer. It is theorized that long-term PPI use can contribute to an increased level of H. Pylori in the digestive tract and possibly, therefore, the increased risk. Although the two research studies arrived at the same conclusion, their findings are not without controversy and have been contradicted by researchers elsewhere.
Heart Attack Risk
In June 2015, the Stanford Medicine journal reported on a data-mining study carried out at Stanford Medical School linking PPIs with an elevated risk of a heart attack. Although researchers were quick to point out that their study did not illustrate a causative link between PPIs and heart attack risk, they stated that the report combed through the health records of nearly 3 million people and found indications that PPI use was associated with a roughly 20% increase in the rate of a subsequent heart-attack in all adult PPI users.
Risk of Fractures of the Hip, Wrist and Spine
The FDA issued a drug safety communication for all PPIs in May 2010, revising the labeling to include safety information about the possibility of increased risk of fractures to the hip, wrist, and spine. The FDA’s decision came on the heels of its own reviews of several studies that reported an increased risk of fractures with PPI use. In fact, the FDA said that some studies found that those of the greatest risk of fracture were those who received high doses of PPIs or used them for a year or more.
Dementia and Cognitive Decline
The Harvard Medical School published an article in 2016 highlighting the findings of a study that shed light on the potential for dementia and cognitive decline with long-term PPI use. Specifically, a 2015 study published in the neurology Journal of the American Medical Association (JAMA) showed a possible association between chronic use of PPIs and dementia risk. Experts compared prescription PPI intake and diagnosis of dementia among approximately 74,000 adults ages 75 and older.
All of the participants in the study were dementia-free at the time that the study commenced. However, follow-up interviews eight years later seemed to indicate that chronic PPI users had a 44% rate of increased risk for dementia. Men were at a slightly higher risk than women and occasional users of PPIs had a much lower risk.
The article publishers and researchers are quick to point out that the study does not mean PPI users will automatically get dementia. It only points to a “statistical association” and the need for ongoing research and discussion about the potential for linkage.
Sources Cited (69)
1) “Proton Pump Inhibitors: Review of Emerging Concerns” https://www.mayoclinicproceedings.org/article/S0025-6196(17)30841-8/fulltext#:~:text=Although%20PPIs%20have%20had%20an,chronic%20kidney%20disease%2C%20and%20dementia.
2) “Risks of PPIs: What’s Real, What Needs More Research” https://healthblog.uofmhealth.org/digestive-health/risks-of-ppis-whats-real-what-needs-more-research
3) “Heartburn Drugs Again Tied to Fatal Risks” https://www.webmd.com/heartburn-gerd/news/20190606/heartburn-drugs-again-tied-to-fatal-risks#1
4) “Long-term Consequences of Chronic Proton Pump Inhibitor Use” https://www.medscape.com/viewarticle/820136
5) “Proton Pump Inhibitors (PPIs)” https://www.aboutgerd.org/medications/proton-pump-inhibitors-ppis.html
6) “Effects of proton pump inhibitors on gastric emptying: a systematic review” https://pubmed.ncbi.nlm.nih.gov/20012198/
7) “Study sounds another warning about proton pump inhibitors” https://discoveries.childrenshospital.org/warning-proton-pump-inhibitors/
8) “Recognizing Proton Pump Inhibitor Risk” https://www.todaysgeriatricmedicine.com/archive/012014p6.shtml
9) “Emerging concerns with PPI therapy” https://www.pharmaceutical-journal.com/opinion/comment/emerging-concerns-with-ppi-therapy/11023415.article?firstPass=false
10) “Long-term Proton Pump Inhibitor Administration Caused Physiological and Microbiota Changes in Rats” https://www.nature.com/articles/s41598-020-57612-8
11) “Impact of pharmacist intervention in minimizing inappropriate use of Proton Pump Inhibitors in the elderly” https://www.caltcm.org/assets/ppi%20poster%20nomura.pdf
12) “Managing GERD with PPIs” https://badgut.org/information-centre/a-z-digestive-topics/managing-gerd-ppis/
13) “Further Evidence to Monitor Long-term Proton Pump Inhibitor Use” https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2755847
14) “REVIEWS IN BASIC AND CLINICAL GASTROENTEROLOGY
AND HEPATOLOGY” https://www.gastrojournal.org/article/S0016-5085(17)35623-8/pdf
15) “GERD guidance for long-term PPI users” https://www.northeastdigestive.com/procedures/gerd-guidance-for-long-term-ppi-users/
16) “Proton pump inhibitors: Review of reported risks and controversies” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6302736/
17) “Heartburn drugs linked to fatal heart and kidney disease, stomach cancer” https://medicine.wustl.edu/news/popular-heartburn-drugs-linked-to-fatal-heart-disease-chronic-kidney-disease-stomach-cancer/
18) “Considering the Risks and Benefits of Proton Pump Inhibitor Use” https://www.oregonclinic.com/about-us/blog/considering-risks-and-benefits-proton-pump-inhibitor-use
19) “PPIs and Acute Interstitial Nephritis” https://www.jwatch.org/jg200606270000001/2006/06/27/ppis-and-acute-interstitial-nephritis
20) “Interstitial nephritis caused by PPIs” https://www.pharmaceutical-journal.com/cpd-and-learning/learning-article/interstitial-nephritis-caused-by-ppis/11117189.article
21) “Proton Pump Inhibitors and Acute Interstitial Nephritis” https://www.cghjournal.org/article/S1542-3565(05)01092-X/pdf
22) “Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury” https://www.kidney-international.org/article/S0085-2538(17)30005-4/fulltext#:~:text=Proton%20pump%20inhibitor%20(PPI)%20use,be%20mediated%20by%20intervening%20AKI.
23) “Proton Pump Inhibitors and Risk of Incident CKD and Progression to ESRD” https://jasn.asnjournals.org/content/27/10/3153
24) “Proton Pump Inhibitors and Risk of Acute and Chronic Kidney Disease: A Retrospective Cohort Study” https://pubmed.ncbi.nlm.nih.gov/30779194/
25) “Acid Reflux and Proton Pump Inhibitors” https://www.kidney.org/atoz/content/acid-reflux-and-proton-pump-inhibitors
26) “Proton Pump Inhibitor Usage and the Risk of Mortality in Hemodialysis Patients” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5932134/
27) “Proton-pump inhibitors for prevention of upper gastrointestinal bleeding in patients undergoing dialysis” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4408464/
28) “Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury” https://www.kidney-international.org/article/S0085-2538(17)30005-4/pdf
29) “PPI Use Linked to Increased Death From CKD, CVD” https://www.renalandurologynews.com/home/news/nephrology/chronic-kidney-disease-ckd/ppi-use-linked-to-increased-death-from-ckd-cvd/
30) “Pantoprazole-induced acute kidney injury: A case report” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5958704/
31) “Common Acid Reflux Medications Linked to Increased Kidney Disease Risk” https://health.ucsd.edu/news/releases/Pages/2019-02-19-common-acid-reflux-medications-linked-to-kidney-disease-risk.aspx
32) “Proton Pump Inhibitors and the Kidney: Implications of Current Evidence for Clinical Practice and When and How to Deprescribe” https://www.ajkd.org/article/S0272-6386(19)30939-4/abstract
33) “Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease” https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2481157
34) “Proton Pump Inhibitors: Considerations With Long-Term Use” https://www.uspharmacist.com/article/proton-pump-inhibitors-considerations-with-longterm-use#:~:text=PPIs%20are%20generally%20well%20tolerated,diarrhea%2C%20nausea%2C%20and%20vomiting.
35) “Chronic kidney disease” https://www.mayoclinic.org/diseases-conditions/chronic-kidney-disease/symptoms-causes/syc-20354521
36) “Proton pump inhibitor-induced hypomagnesemia: A new challenge” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3782221/#:~:text=Hypomagnesemia%20has%20recently%20been%20recognized,the%20gastrointestinal%20absorption%20of%20magnesium.
37) “Migraine and Magnesium” https://www.dizziness-and-balance.com/disorders/central/migraine/treatments/magnesium.html
38) “What’s the Best Time to Take Magnesium?” https://www.healthline.com/nutrition/best-time-to-take-magnesium
39) “Persistent severe hypomagnesemia caused by proton pump inhibitor resolved after laparoscopic fundoplication” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5645624/
40) “Proton pump inhibitors and hypomagnesemia: a rare but serious complication” https://www.kidney-international.org/article/S0085-2538(15)55801-8/fulltext
41) “Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5643276/
42) “FDA Drug Safety Communication: Clostridium difficile associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs)” https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-clostridium-difficile-associated-diarrhea-can-be-associated-stomach
43) “Clostridium difficile” http://www.rdash.nhs.uk/wp-content/uploads/2014/03/WZT766-DP7322-Clostridium-difficile-Booklet-web.pdf
44) “Does the use of proton pump inhibitors (PPIs) increase the risk for Clostridium difficile (C diff) colitis?” https://www.medscape.com/answers/186458-154809/does-the-use-of-proton-pump-inhibitors-ppis-increase-the-risk-for-clostridium-difficile-c-diff-colitis
45) “Association between gastric acid suppression and risk of primary C. diff infection” https://www.mayoclinic.org/medical-professionals/digestive-diseases/news/association-between-gastric-acid-suppression-and-risk-of-primary-c-diff-infection/mac-20429450
46) “How Might PPIs Promote C difficile infection?” https://journalsblog.gastro.org/how-might-ppis-promote-c-difficile-infection/
47) “Association Between Proton Pump Inhibitor Therapy and Clostridium difficile Infection in a Meta-Analysis” https://www.cghjournal.org/article/S1542-3565(11)01078-0/pdf
48) “The Final Word on Proton Pump Inhibitors and Osteoporosis?” https://www.gastrojournal.org/article/S0016-5085(13)00095-4/fulltext#:~:text=In%20September%202012%2C%20the%20US,for%20osteoporosis%2Drelated%20fractures%20of
49) “Proton Pump Inhibitors and Fracture Risk: A Review of Current Evidence and Mechanisms Involved” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6540255/
50) “The Association Between Prolonged Proton Pump Inhibitors Use and Bone Mineral Density” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6914803/#:~:text=PPIs%20are%20generally%20considered%20safe,osteoporosis%20and%20fractures%20remains%20unproven.
51) “Proton Pump Inhibitor (PPI) Therapy and Bone Fractures” https://badgut.org/information-centre/a-z-digestive-topics/ppi-therapy-and-bone-fractures/
52) “By the way, doctor: Does long-term use of Prilosec cause stomach cancer?” https://www.health.harvard.edu/diseases-and-conditions/by_the_way_doctor_does_long-term_use_of_prilosec_cause_stomach_cancer
53) “Can long-term use of acid reflux drugs cause cancer?” https://www.cancercenter.com/community/blog/2019/06/long-term-use-acid-reflux-drugs-cancer
54) “Proton pump inhibitors may mask early gastric cancer” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1114430/
55) “Proton pump inhibitor: The dual role in gastric cancer” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6506576/
56) “Proton pump inhibitors linked to fatal cases of CVD, CKD, stomach cancer” https://www.cardiovascularbusiness.com/topics/vascular-endovascular/ppis-linked-fatal-cases-cvd-ckd-stomach-cancer
57) “Acid reflux drugs linked to increased stomach cancer risk” https://www.nhs.uk/news/cancer/acid-reflux-drugs-linked-increased-stomach-cancer-risk/
58) “PPI use associated with risk of stroke, myocardial infarction” https://www.clinicaladvisor.com/home/topics/cardiovascular-disease-information-center/ppi-use-associated-with-risk-of-stroke-myocardial-infarction/#:~:text=High%2Ddose%20PPI%20correlated%20with,the%20Journal%20of%20Internal%20Medicine.
59) “Abstract 18462: Proton Pump Inhibitor Use Increases the Associated Risk of First-Time Ischemic Stroke. A Nationwide Cohort Study” https://www.ahajournals.org/doi/abs/10.1161/circ.134.suppl_1.18462
60) “Popular heartburn medication may increase ischemic stroke risk” https://www.sciencedaily.com/releases/2016/11/161115163944.htm
61) “Outcome of stroke patients on clopidogrel plus proton-pump inhibitors: a single-center cohort study” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6464665/
62) “Association Between Proton Pump Inhibitor Use and Risk of Stroke” https://www.gastrojournal.org/article/S0016-5085(18)34651-1/fulltext
63) “Proton Pump Inhibitors Linked to Risk for Heart Failure, Death in CAD” https://www.thecardiologyadvisor.com/home/topics/heart-failure/proton-pump-inhibitors-linked-to-risk-for-heart-failure-death-in-cad/#:~:text=Proton%20pump%20inhibitor%20use%20is,study%20published%20in%20PLoS%20One.
64) “Pharmacovigilance: Proton pump inhibitors are associated with increased risk of heart attack” https://www.pharmaceutical-journal.com/news-and-analysis/proton-pump-inhibitors-are-associated-with-increased-risk-of-heart-attack/20068748.article
65) “Some heartburn drugs may boost risk of heart attack, study finds” https://med.stanford.edu/news/all-news/2015/06/some-heartburn-drugs-may-boost-risk-of-heart-attack-study-finds.html
66) “Certain Heartburn Drugs Linked to Increased Risk of Heart Attack” https://www.scientificamerican.com/article/certain-heartburn-drugs-linked-to-increased-risk-of-heart-attack/
67) “Proton pump inhibitor use and risk of dementia” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6408083/
68) “Can a heartburn drug cause cognitive problems?” https://www.health.harvard.edu/blog/can-heartburn-medication-cause-cognitive-problems-201603219369
69) “Dementia Link to Long-Term Proton Pump Inhibitor Use Clarified” https://www.genengnews.com/news/dementia-link-to-long-term-proton-pump-inhibitor-use-clarified/
Proton Pump Inhibitors Lawsuits and Updates
Last Updated October 26, 2020
Proton Pump Inhibitors (PPIs) have been aggressively marketed and prescribed to the American public for many years as a potent tool in the fight against Gastro-Esophageal Reflux Disease (GERD) and a host of other digestive disorders. PPI drugs like Prilosec have been a runaway success for global pharmaceutical giants like AstraZeneca, PLC, accounting for over $26 billion in revenue. AstraZeneca liked Prilosec so much, in fact, that as soon as it fell out from under patent protection, it rolled out another PPI just like Prilosec, called Nexium (marketed as “The Purple Pill”) which went on to generate another $48 billion for the firm.

However, years after entering the market and after many millions of doses, clouds have emerged on the PPI horizon. Cumulative medical journal and scholarly studies published over several decades have associated long-term use of PPIs with kidney injuries. Furthermore, in 2014 the U.S. Food and Drug Administration (FDA) required prescription PPIs to carry warnings on their labels concerning Acute Interstitial Nephritis. Against this backdrop of increasingly dire scientific reporting, nearly 14,000 lawsuits alleging that PPIs have caused renal disease and related injuries have been consolidated into one massive multidistrict litigation in federal court in New Jersey (MDL-2789).
PPIs and Potential for Kidney Damage
Patients who take PPIs have demonstrated an increased risk of kidney damage that could lead to kidney failure. Researchers at the University of Buffalo School of Pharmacy have published studies showing that PPI users have a “four-fold” increase in their risk of acute kidney injuries which, in turn, can lead to more consequential injuries such as Chronic Kidney Disease.
Acute Interstitial Nephritis and Chronic Kidney Disease
PPIs have been specifically associated with a condition known as Acute Interstitial Nephritis (AIN) – a kidney disorder in which the spaces between the kidney tubules become swollen (inflamed). The risk of developing AIN from drugs such as PPIs increases over time due to prolonged use. Prolonged AIN symptoms can progress to Chronic Kidney Disease (CKD) a condition that carries with it a substantially increased risk of death and cardiovascular events.
End-Stage Renal Disease
End-Stage Renal Disease (ESRD) is typically the next stage toward complete renal failure following long-term suffering with CKD. Essentially, a sufferer’s kidneys are no longer able to function properly in a way that the body requires. Treatment for ESRD requires either frequent dialysis or a kidney transplant for the patient to survive.
Lawsuits and Allegations
Complaints have been filed in courts across the United States, many of which have been consolidated in MDL-2789, by people suffering from a range of renal maladies including AIN, CKD, and ESRD who also took either prescription or over-the-counter (OTC) versions of PPIs (or both). In their complaints, many sufferers allege that manufacturers knew of the risks of renal damage from PPIs (in particular Nexium) going back to the late 1980s and that research as far back as 1992 associated PPIs with kidney damage. Furthermore, they claim, studies by the Yale School of Medicine in 2006 made findings of a connection between PPIs, AIN, and CKD in most patients. Some of the claims also reference a 2016 study in the Journal of the American Society of Nephrology that found that PPI use, including Nexium, was independently associated with 20%-to-50% higher risk for CKD.
Sufferers allege that, with all of the mounting scientific evidence compiling, that companies like AstraZeneca and other manufacturers either knew or should have known of the increased risk for CKD posed by PPIs. They claim that manufacturers instead took no action to warn doctors or patients of the risk of kidney disease – and even continued to claim that PPIs such as Nexium did not have any associated risks of kidney injury.
Lawsuit Profiles
Hope Butler
A resident of Ohio, Ms. Butler was among the very first to file a PPI lawsuit naming AstraZeneca in the U.S. District Court for the Southern District of Ohio in March 2017. She states in her complaint that she used prescription Nexium between 2010 and December 2016. Ms. Butler filed her lawsuit after her diagnosis with CKD in March 2015 and her discovery of the increased potential for kidney disease associated with PPIs.
Cheryl Lear
Cheryl Lear, a chronic sufferer with GERD, nonsteroidal anti-inflammatory drug-induced gastropathy, and other peptic disorders from Duval County, Florida, filed a lawsuit naming AstraZeneca, Wyeth Pharmaceuticals, and Pfizer, Inc. (among others) in federal court in 2017. She began taking prescription Prilosec in 2012 and later prescription Protonix through September 2013. Ms. Lear filed her lawsuit after suffering several years with acute kidney injuries, culminating with CKD, and upon learning of the potential connection with her long term use of Prilosec and Protonix
Lawsuits Are Still Being Filed
Individuals who have taken either a prescription or OTC PPIs and have suffered from kidney damage or related illnesses may be able to take legal action. Although each potential case is unique, types of compensation sought typically include medical expenses, loss of income, and pain and suffering. Case reviews are free and do not involve any obligation whatsoever.
Sources Cited (37)
1. “In re: Proton Pumps Inhibitor Litigation (MDL-2789)” https://ecf.jpml.uscourts.gov/doc1/8501834105
2. “Hope Butler v. AstraZeneca Pharmaceuticals, LP, et al.” https://ecf.ohsd.uscourts.gov/doc1/14316373880
3. “Cheryl Lear v. AstraZeneca Pharmaceuticals, LP. et al.” https://ecf.flmd.uscourts.gov/doc1/047117162494
4. “Kidney Damage Tied to Proton Pump Inhibitors” https://www.renalandurologynews.com/home/news/nephrology/acute-kidney-injury/kidney-damage-tied-to-proton-pump-inhibitors/#:~:text=A%20large%20retrospective%20study%20found,a%20large%20population%2Dbased%20study.
5. “Acute interstitial nephritis due to proton pump inhibitors” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3741979/
6. “Proton Pump Inhibitors and CKD” https://jasn.asnjournals.org/content/27/10/2926
7. “Popular heartburn drugs linked to gradual yet ‘silent’ kidney damage” https://medicine.wustl.edu/news/popular-heartburn-drugs-linked-gradual-yet-silent-kidney-damage/
8. “Proton Pump Inhibitor Use and Risk of Chronic Kidney Disease” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4772730/
9. “Chronic PPI Use Linked to Declining Kidney Function in Chronic Kidney Disease” https://www.pharmacytimes.com/news/chronic-ppi-use-linked-to-declining-kidney-function-in-chronic-kidney-disease
10. “Diagnosis and Management of Acute Interstitial Nephritis” https://www.aafp.org/afp/2003/0615/p2527.html#:~:text=Acute%20interstitial%20nephritis%20(AIN)%20defines,edema%20of%20the%20renal%20interstitium.
11. “Interstitial nephritis” https://medlineplus.gov/ency/article/000464.htm
12. “PPIs and kidney disease: from AIN to CKD” https://pubmed.ncbi.nlm.nih.gov/27072818/
13. “End Stage Renal Disease” https://pubmed.ncbi.nlm.nih.gov/27072818/
14. “The physiological background behind and course of development of the first proton pump inhibitor” https://pubmed.ncbi.nlm.nih.gov/25857639/
15. “Harvard Health Letter: Proton-pump inhibitors” https://www.health.harvard.edu/diseases-and-conditions/proton-pump-inhibitors
16. “MedlinePlus: Proton Pump Inhibitors” https://medlineplus.gov/ency/patientinstructions/000381.htm
17. “Healthline: Proton Pump Inhibitors” https://www.healthline.com/health/gerd/proton-pump-inhibitors#1
18. “The Safety of Appropriate Use of Over-the-Counter Proton Pump Inhibitors: An Evidence-Based Review and Delphi Consensus” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5357248/
19. “What you should know about: PPIs” https://www.health.harvard.edu/diseases-and-conditions/what-you-should-know-about-ppis#:~:text=PPIs%20work%20by%20inhibiting%20certain,over%20a%20period%20of%20time.
20. “Proton Pump Inhibitors: Use in Adults” https://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Pharmacy-Education-Materials/Downloads/ppi-adult-factsheet11-14.pdf
21. “25 Years of Proton Pump Inhibitors: A Comprehensive Review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5221858/
22. “Mayo Clinic: Omeprazole (Oral Route)” https://www.mayoclinic.org/drugs-supplements/omeprazole-oral-route/description/drg-20066836
23. “Mayo Clinic Proceedings: Proton Pump Inhibitors: Review of Emerging Concerns” https://www.mayoclinicproceedings.org/article/S0025-6196(17)30841-8/fulltext#:~:text=Although%20PPIs%20have%20had%20an,chronic%20kidney%20disease%2C%20and%20dementia.
24. “The risks of long-term use of proton pump inhibitors: a critical review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6463334/
25. “Advantages and Disadvantages of Long-term Proton Pump Inhibitor Use” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5885718/
26. “Pharmacokinetic Drug Interaction Profiles of Proton Pump Inhibitors: An Update” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3975086/
27. “Drug-drug interaction profiles of proton pump inhibitors” https://pubmed.ncbi.nlm.nih.gov/20608754/
28. “Proton Pump Inhibitors: Considerations With Long-Term Use” https://www.uspharmacist.com/article/proton-pump-inhibitors-considerations-with-longterm-use
29. “FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors” https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-possible-increased-risk-fractures-hip-wrist-and-spine-use-proton-pump#:~:text=FDA%20has%20determined%20an%20osteoporosis,dose%20PPI%20use%20is%20unlikely.
30. “FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of Proton Pump Inhibitor drugs (PPIs)” https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-low-magnesium-levels-can-be-associated-long-term-use-proton-pump
31. “Forbes Magazine: Hard to Swallow” https://www.forbes.com/forbes/2002/0513/199.html#3a838e544282
32. “American Council on Science and Health: Nexium: The Dark Side Of Pharma” https://www.acsh.org/news/2017/01/18/nexium-dark-side-pharma-10546
33. “Is the concomitant use of clopidogrel and Proton Pump Inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: a systematic review and meta-analysis of recently published studies (2012 – 2016)” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5221663/#:~:text=Because%20PPIs%20can%20act%20as,increase%20in%20adverse%20clinical%20outcomes.
34. “Harvard Medical School – Trends in Medicine: The Link Between Proton Pump Inhibitors and Kidney Disease” https://trends.hms.harvard.edu/2017/04/27/the-link-between-proton-pump-inhibitors-and-kidney-disease/#:~:text=In%20a%20recent%20meta%2Danalysis,1.07%2D1.72%2C%20respectively).
35. “Association of Long-term Proton Pump Inhibitor Therapy with Bone Fractures and effects on Absorption of Calcium, Vitamin B12, Iron, and Magnesium” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2974811/
36. “Harvard Women’s Health Watch: By the way, doctor: Does long-term use of Prilosec cause stomach cancer?” https://www.health.harvard.edu/diseases-and-conditions/by_the_way_doctor_does_long-term_use_of_prilosec_cause_stomach_cancer#:~:text=This%20has%20raised%20some%20concerns,increased%20risk%20for%20stomach%20cancer.
37. “FDA Warns About Bone Breaks And Heartburn Drugs” https://www.npr.org/sections/health-shots/2010/05/26/127132723/fda-warns-about-bone-risks-from-heartburn-drugs
Drug Law Journal's publishing and research are sponsored by the DDP Injury Law Group in Washington, D.C. Their legal team is focused on protecting the rights of injury victims.
Furthermore, they understand and appreciate the importance of a trusted attorney-client relationship.
The DDP Injury Law Group uses their years of experience with litigation to ensure their clients can fight for the compensation they deserve.
Always seek the advice of a medical professional when making personal health choices.
The Offices of DrugLawJournal.com are located at:
1800 North Orange Avenue, Suite C
Orlando, Florida 32804
DrugLawJournal.com is sponsored by the DDP Injury Law Group, and therefore may be considered attorney advertising. The information contained on DrugLawJournal.com is provided for informational purposes only, and should not be construed as legal or medical advice on any subject matter. No viewers of this site should discontinue taking a prescribed medication on the basis of any information on this site and should always first consult with a doctor concerning any medication. Viewers should understand that if they refrain from taking prescribed medication without appropriate medical advice they can suffer injury or death.
No viewers of content from this site, clients or otherwise, should act or refrain from acting on the basis of any content included in the site without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from an attorney licensed in the viewer’s state. Viewing information from DrugLawJournal.com does not create an attorney-client relationship between you and DDP Injury Law Group or DrugLawJournal.com nor is it intended to do so.The content of DrugLawJournal.com may not reflect current legal developments, verdicts or settlements. Prior results do not predict a similar outcome. For more information, please visit our web site’s disclaimer.
©2025 DrugLawJournal.com | Privacy Policy | Terms & ConditionsStay Informed
Sign up to receive peroidic updates from our expert team of researchers, highlighting defective drugs, devices, and legal issues related to your health.





